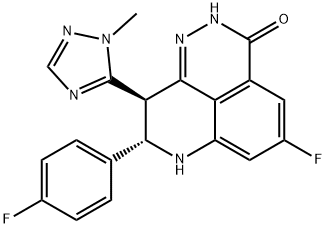
COMPUTED DESCRIPTORS
| Molecular Weight | 380.4 g/mol |
|---|---|
| XLogP3 | 2.3 |
| Hydrogen Bond Donor Count | 2 |
| Hydrogen Bond Acceptor Count | 7 |
| Rotatable Bond Count | 2 |
| Exact Mass | 380.11971542 g/mol |
| Monoisotopic Mass | 380.11971542 g/mol |
| Topological Polar Surface Area | 84.2 Ų |
| Heavy Atom Count | 28 |
| Formal Charge | 0 |
| Complexity | 654 |
| Isotope Atom Count | 0 |
| Defined Atom Stereocenter Count | 2 |
| Undefined Atom Stereocenter Count | 0 |
| Defined Bond Stereocenter Count | 0 |
| Undefined Bond Stereocenter Count | 0 |
| Covalently-Bonded Unit Count | 1 |
| Compound Is Canonicalized | Yes |
PRODUCT INTRODUCTION
description
Talazoparib was approved by the FDA for use in germline BRCA mutated, HER2 negative, locally advanced or metastatic breast cancer on October 16, 2018 under the trade name Talzenna. Talzenna was granted approval based on the results of the EMBRACA trial in which talazoparib resulted in a mean 8.6 months progression-free survival time versus physician's choice chemotherapy which resulted in 5.6 months progression-free survival.