Bleomycin sulfate
Synonym(s):Blenoxane;Bleo;Bleomycin Sulfate, Streptomyces verticillus - CAS 9041-93-4 - Calbiochem;Blexane
- CAS NO.:9041-93-4
- Empirical Formula: C110H168N34O46S7
- Molecular Weight: 2927.17
- MDL number: MFCD00070310
- EINECS: 232-925-2
- SAFETY DATA SHEET (SDS)
- Update Date: 2024-12-10 10:05:43
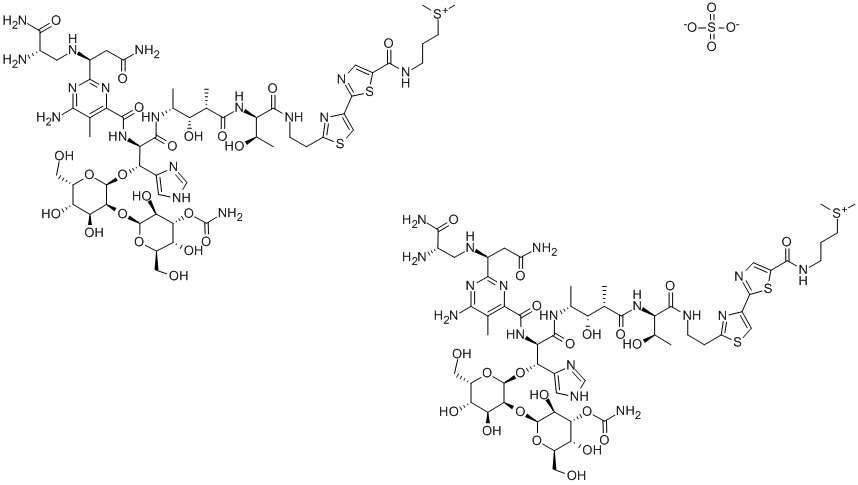
What is Bleomycin sulfate?
Description
Bleomycin sulfate (9041-93-4) coordinates with metals producing reactive oxygen species which causes oxidative damage to DNA1?and RNA2. Induces double-strand DNA damage.3?Commonly used to induce lung fibrosis in animal disease models.4,5?Anticancer agent in clinical use.6
Chemical properties
White Powder
The Uses of Bleomycin sulfate
Bleomycin sulfate binds to DNA, and can inhibit DNA synthesis and causes scission of DNA. Cleaves DNA, requires binding to oxygen and a metal ion, such as copper or iron. Able to cleave RNA, but at less and more selective degree. Also reported to induce and regulate apoptosis in a variety of cells, and inhibit tumor angiogenesis.
The Uses of Bleomycin sulfate
A group of related glycopeptide antibiotics. Bleomycin A2 is the main component of the bleomycin employed clinically. Antineoplastic
The Uses of Bleomycin sulfate
Bleomycin is a complex of 11 glycopeptide antitumour antibiotics originally isolated from Streptomyces verticillus in 1972. The dominant components of the complex are bleomycin A2 and B2, which typically represent >90% of the total weight . Bleomycins have found clinical application in the treatment of a range of tumours. Bleomycins act by intercalation of DNA and RNA. In the presence of oxygen and metal ions, notably copper and iron, bleomycins form a pseudo-enzyme that induces DNA cleavage.
The Uses of Bleomycin sulfate
Bleomycin is a complex of 11 glycopeptide antitumor antibiotics originally isolated from Streptomyces verticillus in 1972. The dominant components of the complex are bleomycin A2 and B2, which typically represent >90% of the total weight. Bleomycins have found clinical application in the treatment of a range of tumors. Bleomycins act by intercalation of DNA and RNA. In the presence of oxygen and metal ions, notably copper and iron, bleomycins form a pseudo-enzyme that induces DNA cleavage.
What are the applications of Application
Bleomycin Sulfate is a glycopeptide antibiotic produced by bacterium S. verticillus which is capable of cleaving DNA backbone
brand name
Blenoxane (Bristol-Myers Squibb).
General Description
Bleomycin occurs as a white powder and is available in 15-and 30-U vials for reconstitution in water. It may be givenintravenously, intramuscularly, or subcutaneously. It is used in the treatment of squamous cell carcinoma of the headneck, cervix, penis, and vulva. It is also used in Hodgkin’sand non-Hodgkin’s lymphoma as well as testicular carcinoma.Unlabeled uses include the treatment of mycosis fungoides,osteosarcoma, and AIDS-related Kaposi sarcoma.
Biochem/physiol Actions
Bleomycin sulfate binds to DNA, causes ssDNA scission at specific base sequences and inhibits DNA synthesis. This inhibitory action requires bleomycin to bind oxygen and a metal ion. It can also cleave RNA, to a lesser degree but more selectively. It acts as an inducer and regulator of apoptosis and inhibits tumor angiogenesis.
Clinical Use
Bleomycin is used IV in the palliative treatment of squamous cell head and neck cancers, testicular and other genital carcinomas, and Hodgkin's and non-Hodgkin's lymphoma.
Side Effects
It is excreted via the kidneys, and serum concentrations of active drug are increased in patients with renal disease. The elimination half-life can rise from 2 to 4 hours to more than 20 hours in renal failure, resulting in significant toxicity, especially pulmonary toxicity.
Veterinary Drugs and Treatments
Bleomycin has occasionally been used as adjunctive treatment of lymphomas, squamous cell carcinomas, teratomas, and nonfunctional thyroid tumors in both dogs and cats. Recent work has demonstrated that bleomycin may be promising for intralesional treatment for a variety of localized tumors with or without concomitant electropermeabilization.
References
1) Petering?et al.?(1990),?The role of redox-active metals in the mechanism of action of bleomycin; Chem. Biol. Interact.,?73?133 2) Huttenhofer?et al. (1992),?Cleavage of tRNA by Fe(II)-bleomycin; J. Biol. Chem.,?267?24471 3) Lee?et al.?(2017)?ASF1a Promotes Non-homologous End Joining Repair by facilitating Phosphorylation of MDC1 by ATM at Double-Strand Breaks;?Mol. Cell?68?61 4)?Xie?et al.?(2016), Upregulation of RGS2: a new mechanism for pirfenidone amelioration of pulmonary fibrosis; Respir. Res.,?17?103 5) Inomata?et al. (2014) Pirfenidone inhibits fibrocyte accumulation in the lungs in bleomycin-induced murine pulmonary fibrosis; Respir. Res.,?15?16 6) Tanaka?et al. (2008)?Increased glutathione level is not involved in enhanced bleomycin sensitivity in cisplatin-resistant 2780CP cells; Anticancer Res.,?28?2663
Properties of Bleomycin sulfate
| Melting point: | 200-204°C (dec.) |
| storage temp. | 2-8°C |
| solubility | H2O: 20 mg/mL |
| form | powder |
| color | white |
| Water Solubility | Soluble in water or DMSO |
| Merck | 14,1318 |
| Stability: | Stable for 1 year from the date of purchase. Solutions in distilled water may be stored at -20°C for up to 3 months. |
| CAS DataBase Reference | 9041-93-4(CAS DataBase Reference) |
Safety information for Bleomycin sulfate
| Signal word | Danger |
| Pictogram(s) |
 Health Hazard GHS08 |
| GHS Hazard Statements |
H340:Germ cell mutagenicity H351:Carcinogenicity |
| Precautionary Statement Codes |
P201:Obtain special instructions before use. P202:Do not handle until all safety precautions have been read and understood. P280:Wear protective gloves/protective clothing/eye protection/face protection. P308+P313:IF exposed or concerned: Get medical advice/attention. P405:Store locked up. P501:Dispose of contents/container to..… |
Computed Descriptors for Bleomycin sulfate
| InChIKey | OOXTWFJZZAJGKA-YWZNXBAZNA-N |
Bleomycin sulfate manufacturer
New Products
(S)-3-Aminobutanenitrile hydrochloride 4-Methylphenylacetic acid N-Boc-D-alaninol N-BOC-D/L-ALANINOL Tert-butyl bis(2-chloroethyl)carbamate 3-Morpholino-1-(4-nitrophenyl)-5,6-dihydropyridin- 2(1H)-one Furan-2,5-Dicarboxylic Acid Tropic acid 1-Bromo-3,5-Di-Tert-Butylbenzene S-2-CHLORO PROPIONIC ACID ETHYL ISOCYANOACETATE 2-Bromo-1,3-Bis(Dimethylamino)Trimethinium Hexafluorophosphate 4-IODO BENZOIC ACID 3-NITRO-2-METHYL ANILINE 1-(2,4-DICHLOROPHENYL) ETHANAMINE (2-Hydroxyphenyl)acetonitrile 4-Bromopyrazole 2-(Cyanocyclohexyl)acetic acid 4-methoxy-3,5-dinitropyridine 1-(4-(aminomethyl)benzyl)urea hydrochloride 2-aminopropyl benzoate hydrochloride diethyl 2-(2-((tertbutoxycarbonyl)amino) ethyl)malonate tert-butyl 4- (ureidomethyl)benzylcarbamate Ethyl-2-chloro((4-methoxyphenyl)hydrazono)acetateRelated products of tetrahydrofuran


You may like
-
 Bleomycin sulfate CAS 9041-93-4View Details
Bleomycin sulfate CAS 9041-93-4View Details
9041-93-4 -
 Bleomycin Sulfate (mixture) CAS 9041-93-4View Details
Bleomycin Sulfate (mixture) CAS 9041-93-4View Details
9041-93-4 -
 Bleomycin sulfate CAS 9041-93-4View Details
Bleomycin sulfate CAS 9041-93-4View Details
9041-93-4 -
 1975-50-4 98%View Details
1975-50-4 98%View Details
1975-50-4 -
 2-HYDROXY BENZYL ALCOHOL 98%View Details
2-HYDROXY BENZYL ALCOHOL 98%View Details
90-01-7 -
 2-Chloro-1,3-Bis(Dimethylamino)Trimethinium Hexafluorophosphate 221615-75-4 98%View Details
2-Chloro-1,3-Bis(Dimethylamino)Trimethinium Hexafluorophosphate 221615-75-4 98%View Details
221615-75-4 -
 14714-50-2 (2-Hydroxyphenyl)acetonitrile 98+View Details
14714-50-2 (2-Hydroxyphenyl)acetonitrile 98+View Details
14714-50-2 -
 118753-70-1 98+View Details
118753-70-1 98+View Details
118753-70-1