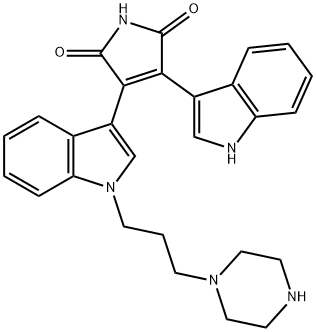
Bisindolylmaleimide VIII acetate salt
- CAS NO.:138516-31-1
- Empirical Formula: C24H22N4O2
- Molecular Weight: 398.464
- MDL number: MFCD00909467
- SAFETY DATA SHEET (SDS)
- Update Date: 2024-11-19 20:33:22
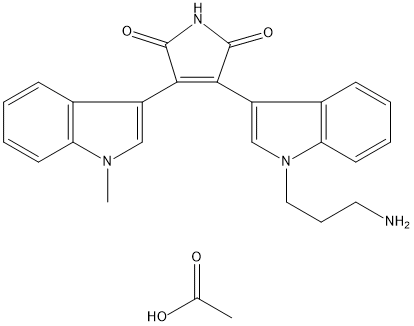
What is Bisindolylmaleimide VIII acetate salt?
The Uses of Bisindolylmaleimide VIII acetate salt
It is a potent inhibitor of protein kinase C (PKC) activity. Bisindolylmaleimide VIII (Bis VIII) has been previously shown to enhance Fas-mediated apoptosis through a protein kinase C-independent mechanism.
What are the applications of Application
Bisindolylmaleimide VIII is a selective PKC inhibitor
Biological Activity
bisindolylmaleimide viii is a protein kinase c (pkc) inhibitor.the protein kinase c (pkc) consists of a family of closely related isoenzymes mediating various signal transduction processes. the ten isoenzymes that have been currently identified can be divided into three families on the basis of their different requirements for activation. members of the conventional pkc family are ca2+ and phospholipid-dependent and are activated by diacylglycerols and phorbol esters.
in vitro
previous study found that bisindolylmaleimides carrying a straight-chain alkyl side-chain bearing a cationic substituent, such as ro 31-7549 (bisindolylmaleimide viii) and ro 31-8220, showed a significant improvement in potency over the simple bisindolylmaleimides. for bisindolylmaleimide viii, a further improvement in potency was obtained by restricting the position of the amine substituent. moreove, unlike the indolocarbazole, staurosporine, which displayed 2-fold selectivity for pkc-β over the other examined isoenzymes, bisindolylmaleimide viii exhibited a slight selectivity for pkc-α over the other isoenzymes. compounds such as bisindolylmaleimide viii carrying a straight-chain alkyl side-chain with the cationic substituent were found to be equipotent as inhibitors of pkc-β, pkc-γ and pkc-ε [1].
in vivo
animal study found that, in neonatal rats, high glucose levels could induce the hypertrophy of cardiomyocytes. ro-31-8220, a analog of bisindolylmaleimide viii, was able to reverse the effect of high glucose on the cardiac myocytes,which might be through pkc/nf-κb/c-fos pathway [2].
References
[1] wilkinson, s. e.,parker, p.j. and nixon, j.s. isoenzyme specificity of bisindolylmaleimides, selective inhibitors of protein kinase c. biochemistry journal 294, 335-337 (1993).
[2] zhang, w. b. et al. reverse effect of protein kinase c inhibitor ro-31-8220 on the hypertrophy of cardiomyocytes of neonatal rats induced by high glucose levels. chinese journal of pathophysiology. 2009-08.
Properties of Bisindolylmaleimide VIII acetate salt
| storage temp. | -20°C |
| solubility | Soluble in DMSO |
| form | Red powder. |
| color | Brown to reddish brown |
Safety information for Bisindolylmaleimide VIII acetate salt
Computed Descriptors for Bisindolylmaleimide VIII acetate salt
New Products
Tert-butyl bis(2-chloroethyl)carbamate 4-Methylphenylacetic acid N-Boc-D-alaninol N-BOC-D/L-ALANINOL 3-Morpholino-1-(4-nitrophenyl)-5,6-dihydropyridin- 2(1H)-one Furan-2,5-Dicarboxylic Acid Tropic acid 1,1’-CARBONYLDIIMIDAZOLE DIETHYL AMINOMALONATE HYDROCHLORIDE R-2-BENZYLOXY PROPIONIC ACID 1,1’-CARBONYLDI (1,2-4 TRIAZOLE) N-METHYL INDAZOLE-3-CARBOXYLIC ACID (2-Hydroxyphenyl)acetonitrile 4-Bromopyrazole 5-BROMO-2CYANO PYRIDINE 5,6-Dimethoxyindanone 5-broMo-2-chloro-N-cyclopentylpyriMidin-4-aMine 2-(Cyanocyclohexyl)acetic acid 4-methoxy-3,5-dinitropyridine 2-aminopropyl benzoate hydrochloride 1-(4-(aminomethyl)benzyl)urea hydrochloride diethyl 2-(2-((tertbutoxycarbonyl)amino) ethyl)malonate tert-butyl 4- (ureidomethyl)benzylcarbamate Ethyl-2-chloro((4-methoxyphenyl)hydrazono)acetateRelated products of tetrahydrofuran

You may like
-
 2033-24-1 98%View Details
2033-24-1 98%View Details
2033-24-1 -
 1975-50-4 98%View Details
1975-50-4 98%View Details
1975-50-4 -
 2-HYDROXY BENZYL ALCOHOL 98%View Details
2-HYDROXY BENZYL ALCOHOL 98%View Details
90-01-7 -
 2-Chloro-1,3-Bis(Dimethylamino)Trimethinium Hexafluorophosphate 221615-75-4 98%View Details
2-Chloro-1,3-Bis(Dimethylamino)Trimethinium Hexafluorophosphate 221615-75-4 98%View Details
221615-75-4 -
 61397-56-6 CIS BROMO BENZOATE 98%View Details
61397-56-6 CIS BROMO BENZOATE 98%View Details
61397-56-6 -
 14714-50-2 (2-Hydroxyphenyl)acetonitrile 98+View Details
14714-50-2 (2-Hydroxyphenyl)acetonitrile 98+View Details
14714-50-2 -
 118753-70-1 98+View Details
118753-70-1 98+View Details
118753-70-1 -
 733039-20-8 5-broMo-2-chloro-N-cyclopentylpyriMidin-4-aMine 98+View Details
733039-20-8 5-broMo-2-chloro-N-cyclopentylpyriMidin-4-aMine 98+View Details
733039-20-8