BIS(1,5-CYCLOOCTADIENE)NICKEL(0)
Synonym(s):Bis(cyclooctadiene)nickel;Ni(COD)2
- CAS NO.:1295-35-8
- Empirical Formula: C16H24Ni
- Molecular Weight: 275.06
- MDL number: MFCD00058902
- EINECS: 215-072-0
- SAFETY DATA SHEET (SDS)
- Update Date: 2025-07-04 15:08:36
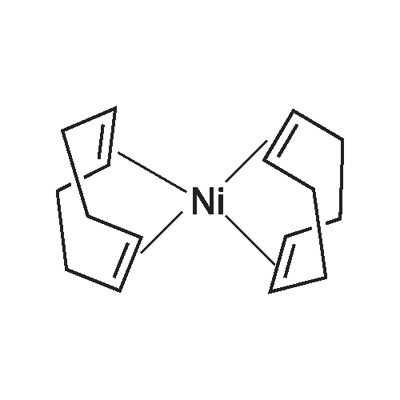
What is BIS(1,5-CYCLOOCTADIENE)NICKEL(0)?
Chemical properties
Light yellow to Brown powder to crystal.
The Uses of BIS(1,5-CYCLOOCTADIENE)NICKEL(0)
Bis(1,5-cyclooctadiene)nickel(0) is used as a catalyst for the cycloaddition of 1,3-dienes and is used to catalyze the addition of allyl phenyl sulfide to alkynes leading to 1,4-dienes. Also, it is involved as a catalyst for asymmetric alpha-arylation and heteroarylation of ketones with chloroarenes, stereoselective borylative ketone-diene coupling, cycloaddition of benzamides with internal alkynes and methyl carboxylation of homopropargylic alcohols.
Preparation
Bis(1,5-cyclooctadiene)nickel(0) is prepared by reduction of anhydrous nickel(II) acetylacetonate in the presence of the diolefin:
Ni(acac)2 + 2 cod + 2 AlEt3 → Ni(cod)2 + 2 acacAlEt2 + C2H6 + C2H4
Ni(cod)2 is moderately soluble in several organic solvents. One or both 1,5-cyclooctadiene ligands are readily displaced by phosphines, phosphites, bipyridine, and isocyanides. If exposed to air, the solid oxidizes to nickel(II) oxide. As a result, this compound is generally handled in a glovebox.
Reactions
Catalyst for Grignard metathesis chain-growth polymerization of Poly(bithienylmethylene)s
Catalyst for neopentylglycolborylation of ortho-substituted aryl halides
Catalyst for Suzuki-Miyaura coupling reactions of heteroaryl ethers with arylboronic acids
Catalyst for carboxylation of naphthyl pivalates with CO2
Catalyst decarboxylative C?P cross-coupling of alkenyl acids with P(O)H compounds
Catalyst for direct amination of phenols via C–O Bond Activation using 2,4,6-Trichloro-1,3,5-triazine as reagent
Catalyst for conversion of aryl, heteroaryl and pharmaceutically relevant chlorides to the corresponding trifluoromethyl sulphides
Catalytic precursor for Suzuki–Miyaura cross-coupling reactions in water under very mild reaction conditions: (a) aryl–heteroaryl cross-couplings; (b) Hetero–heteroaryl cross-couplings

General Description
Yellow crystals or yellowish green solid.
Reactivity Profile
BIS(1,5-CYCLOOCTADIENE)NICKEL(0) is extremely air and moisture sensitive. BIS(1,5-CYCLOOCTADIENE)NICKEL(0) is sensitive to exposure to light. BIS(1,5-CYCLOOCTADIENE)NICKEL(0) is incompatible with oxidizing agents, acids and acid fumes.
Fire Hazard
Flash point data for BIS(1,5-CYCLOOCTADIENE)NICKEL(0) are not available. BIS(1,5-CYCLOOCTADIENE)NICKEL(0) is probably combustible.
Purification Methods
It is available in sealed ampoules under N2. All procedures should be carried out in a dry box and in an atmosphere of N2 or Argon in subdued light because the complex is light and oxygen sensitive, and flammable. The solid is washed with dry Et2O (under Ar) and separates from toluene as yellow crystals. Filter this under Ar gas pressure, place the crystals in a container and dry under a vacuum of 0.01mm to remove adhered toluene, flush with Ar and seal them under Ar or N2 in glass ampoules. [Semmelhack Org Reactions 19 115 and 178 1972, Wilke et al. Justus Liebigs Ann Chem 699 1 1966, Wender & Jenkins J Am Chem Soc 111 6432 1989, Fieser & Fieser Reagents for Org Synth 4 33, 16 29, 17 32.] SUSPECTED CARCINOGEN.
Properties of BIS(1,5-CYCLOOCTADIENE)NICKEL(0)
| Melting point: | 60 °C (dec.)(lit.) |
| storage temp. | -20°C |
| solubility | Benzene (Partially Dissolved), THF (Slightly) |
| form | Shiny Crystalline Powder |
| color | Gold |
| Water Solubility | Soluble in benzene, toluene, terahydrofuran, ether, dimethyl formamide, hexamethylphosphoramide, N-methylpyrrolidinone. Insoluble in water. |
| Sensitive | Air Sensitive |
| Exposure limits | NIOSH: IDLH 10 mg/m3; TWA 0.015 mg/m3 |
| Stability: | Air Sensitive |
| CAS DataBase Reference | 1295-35-8(CAS DataBase Reference) |
| NIST Chemistry Reference | Nickel, bis[(1,2,5,6-«eta»)-1,5-cyclooctadiene]-(1295-35-8) |
| EPA Substance Registry System | Bis[(1,2,5,6-.eta.)-1,5-cyclooctadiene]nickel (1295-35-8) |
Safety information for BIS(1,5-CYCLOOCTADIENE)NICKEL(0)
| Signal word | Danger |
| Pictogram(s) |
 Flame Flammables GHS02  Exclamation Mark Irritant GHS07  Health Hazard GHS08 |
| GHS Hazard Statements |
H228:Flammable solids H317:Sensitisation, Skin H351:Carcinogenicity H372:Specific target organ toxicity, repeated exposure |
| Precautionary Statement Codes |
P201:Obtain special instructions before use. P202:Do not handle until all safety precautions have been read and understood. P210:Keep away from heat/sparks/open flames/hot surfaces. — No smoking. P280:Wear protective gloves/protective clothing/eye protection/face protection. P302+P352:IF ON SKIN: wash with plenty of soap and water. P308+P313:IF exposed or concerned: Get medical advice/attention. |
Computed Descriptors for BIS(1,5-CYCLOOCTADIENE)NICKEL(0)
New Products
Indole Methyl Resin tert-butyl 9-methoxy-3-azaspiro[5.5]undecane-3-carboxylate Boc-His(Boc)-OH 2-CTC Resin 4-Chloro-7-tosy1-7Hpyrrolo[2,3-d]pyrimidine 5,7-Dibromo-1H-indole 2,5-dichloro-N-hydroxy-4,6-dimethylpyridine-3-carboximidamide 2,2-Dimethoxy-7-azaspiro[3.5]nonane hydrochloride 4-chloromethyl-5-methyl-1,3-dioxol-2-one (DMDO-Cl) R-2-BENZYLOXY PROPIONIC ACID 1,1’-CARBONYLDIIMIDAZOLE 1,1’-CARBONYLDI (1,2-4 TRIAZOLE) N-METHYL INDAZOLE-3-CARBOXYLIC ACID 4-((2-hydroxyethyl)thio)benzoic acid 1-(TERT-BUTOXYCARBONYL)-2-PYRROLIDINONE Methyl 6-methylnicotinate 3-Pyridineacrylic acid tert-Butyl carbazate TETRAHYDRO-2H-PYRAN-3-OL 2-((4-morpholinophenylamino) (methylthio) methylene) malononitrile 3-(4-morpholinophenylamino)-5-amino-1H-pyrazole-4-carbonitrile 2,4-dihydroxybenzaldehyde 1,3-Diethyl-1,3-Diphenylurea Methyl 2-methylquinoline-6-carboxylateRelated products of tetrahydrofuran
You may like
-
 1295-35-8 Bis(1,5-cyclooctadiene)nickel 98%View Details
1295-35-8 Bis(1,5-cyclooctadiene)nickel 98%View Details
1295-35-8 -
 Bis(1,5-cyclooctadiene)nickel(0) CAS 1295-35-8View Details
Bis(1,5-cyclooctadiene)nickel(0) CAS 1295-35-8View Details
1295-35-8 -
 Bis(1,5-cyclooctadiene)nickel(0) CAS 1295-35-8View Details
Bis(1,5-cyclooctadiene)nickel(0) CAS 1295-35-8View Details
1295-35-8 -
 Bis(1,5-cyclooctadiene)nickel(0) (Wax encapsulated) (ca. 0.05mmol/capsule) CAS 1295-35-8View Details
Bis(1,5-cyclooctadiene)nickel(0) (Wax encapsulated) (ca. 0.05mmol/capsule) CAS 1295-35-8View Details
1295-35-8 -
 Bis(1,5-cyclooctadiene)nickel(0) CAS 1295-35-8View Details
Bis(1,5-cyclooctadiene)nickel(0) CAS 1295-35-8View Details
1295-35-8 -
 Bis(1,5-cyclooctadiene)nickel(0) CAS 1295-35-8View Details
Bis(1,5-cyclooctadiene)nickel(0) CAS 1295-35-8View Details
1295-35-8 -
 Bis (1,5-Cyclooctadiene) Nickel(0), For LaboratoryView Details
Bis (1,5-Cyclooctadiene) Nickel(0), For LaboratoryView Details
1295-35-8 -
 Thiourea 99% ARView Details
Thiourea 99% ARView Details
62-56-6