Biguanide
- CAS NO.:56-03-1
- Empirical Formula: C2H7N5
- Molecular Weight: 101.11
- MDL number: MFCD00045592
- EINECS: 2002518
- SAFETY DATA SHEET (SDS)
- Update Date: 2024-12-18 13:37:16
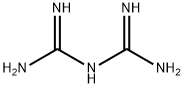
What is Biguanide?
Description
French physician Jean Sterne published the first clinical trial in 1957 suggesting metformin as a treatment for diabetes. The United Kingdom introduced this drug in 1958 followed by Canada in 1972, and the United States in 1995. As of 2010, metformin was one of only two oral antidiabetics in the World Health Organization model list of essential medicines, the other being glibenclamide. Metformin is believed to enjoy the popularity of being the most widely prescribed antidiabetic drug in the world. It has been estimated that the United States alone has filled nearly a million prescriptions in 2010 for its generic formulations. Biguanides are not hypoglycemic agents; rather they promote euglycemia (antihyperglycemic). Biguanides are used both as monotherapy and in combination with other oral hypoglycemic agents to control hyperglycemia. Although very safe in most instances, the major toxicity from acute or chronic use of biguanides has been reported to be lactic acidosis. In fact, the high rate of severe lactic acidosis was the only major cause of withdrawal of phenformin from the US market in 1976.
Chemical properties
Biguanide [56-03-1], C2H7N5, Mr 101.11, mp 130 ℃ (which decomposes violently at 142 ℃), is a moderately strong base (pKb 2.9). It is readily soluble in water and ethanol, and insoluble in ether, benzene, and chloroform. The free base decomposes gradually in aqueous solution. Biguanide forms stable salts with most acids.
The Uses of Biguanide
Biguanides are used as an oral drug for the management of mild to moderately severe noninsulin-dependent diabetes mellitus, or NIDDM, (Type II) in obese or overweight patients who are usually above 40 years of age. It is important that for the administration of this drug the disease should have adult onset. Polymeric biguanides were originally developed as a presurgery antimicrobial scrub and in 1977, it was introduced in the market for treating pools and spas as a disinfectant under the trade name Baquacil. The US Environmental Protection Agency approved this agent as the only nonhalogen sanitizer of pools and spas. Biguanide itself is combined with algaecides and hydrogen peroxide for periodic oxidation of pools and spas. Biguanides are incompatible with chlorine, ozone, detergents, and ionizers, but are compatible with water ion balancing chemicals. Biguanide in the form of polyaminopropyl biguanide serves as a disinfectant, and preservative for skin disinfection, contact lens cleaning solutions, and deodorant body sprays. Biguanides reduce the surface tension of water, which gives it a smoother feeling. They are stable in sunlight and temperature. At recommended concentrations when used in pools and spas, biguanides do not irritate the skin or eyes and do not corrode the pool equipment.
Background
Biguanide has been investigated for the treatment of Diabetes Mellitus.
Definition
ChEBI: Biguanide is a member of guanidines.
Environmental Fate
Globally, hundreds of millions of patients are prescribed this drug annually. Metformin was discovered before the era of target-based drug discovery, and its molecular mechanism of action remains an important focus of diabetes research. Advances in our understanding of metformin’s molecular targets are likely to enable target-based identification of secondgeneration drugs with similar properties, a development that has been a difficult task until now. Besides its potent antidiabetic properties, Metformin’s potential as a targeted anticancer agent is being explored in a number of laboratories throughout the world.
Metabolism
Not Available
Purification Methods
Crystallise biguanide from EtOH. It gives a red Cu derivative, and it forms salts with many metals. The monohydrochloride has m 235o [38664-03-8] and the dihydrochloride forms plates with m 248o (213-214o, also reported) [25836-74-2]. [Beilstein 3 H 93, 3 I 44, 3 II 76, 3 III 171, 3 IV 162.]
Toxicity evaluation
Biguanides, salts of biguanide, or biguanide-like compounds such as Metformin hydrochloride production and use as an antidiabetic medication may result in its release to the environment through various waste streams. Metformin hydrochloride is expected to exist in the dissociated form as metformin in the environment. If released to air, an estimated vapor pressure of 0.0034 mm Hg at 25 °C indicates metformin will exist solely in the vapor phase in the ambient atmosphere. Metformin, when in vapor phase, is expected to be degraded in the atmosphere by reaction with photochemically-produced hydroxyl radicals; the half-life for this reaction in air is estimated to be 15 min. If released to soil, metformin is expected to have high mobility based on an estimated Koc of 110. Volatilization from moist soil surfaces is not expected to be an important fate process based on an estimated Henry’s law constant of 7.6×10-16 atm-cu m mol-1. The pKa of metformin is 12.4, indicating that this compound will primarily exist in cation form in the environment, and cations generally adsorb to organic carbon and clay more strongly than their neutral counterparts. Based on metformin’s vapor pressure, it is not expected to volatilize from dry soil surfaces. Based on the estimated Koc of metformin, it is not expected to adsorb to suspended solids and sediment if released into water. Volatilization from water surfaces is not expected to be an important fate process based on this compound’s estimated Henry’s law constant. Furthermore, a pKa of 12.4 indicates metformin will exist almost entirely in the ionized form at pH values of 5–9. An estimated bioconcentration factor of 3.2 suggests the potential for bioconcentration in aquatic organisms is low. Hydrolysis is not expected to be an important environmental fate process since this compound lacks functional groups that hydrolyze under environmental conditions. Occupational exposure to metformin hydrochloride may occur through inhalation and dermal contact with this compound at workplaces where metformin hydrochloride is produced or used.
Properties of Biguanide
| Melting point: | 130° |
| Boiling point: | 189.3±23.0 °C(Predicted) |
| Density | 1.80±0.1 g/cm3(Predicted) |
| refractive index | 1.7100 (estimate) |
| pka | 11.52, 2.93(at 25℃) |
| CAS DataBase Reference | 56-03-1 |
| EPA Substance Registry System | Imidodicarbonimidic diamide (56-03-1) |
Safety information for Biguanide
Computed Descriptors for Biguanide
New Products
Tert-butyl bis(2-chloroethyl)carbamate 4-Methylphenylacetic acid N-Boc-D-alaninol N-BOC-D/L-ALANINOL N-octanoyl benzotriazole 3-Morpholino-1-(4-nitrophenyl)-5,6-dihydropyridin- 2(1H)-one Furan-2,5-Dicarboxylic Acid DIETHYL AMINOMALONATE HYDROCHLORIDE 1,1’-CARBONYLDIIMIDAZOLE R-2-BENZYLOXY PROPIONIC ACID 1,1’-CARBONYLDI (1,2-4 TRIAZOLE) N-METHYL INDAZOLE-3-CARBOXYLIC ACID (2-Hydroxyphenyl)acetonitrile 4-Bromopyrazole 5-BROMO-2CYANO PYRIDINE 5,6-Dimethoxyindanone 5-broMo-2-chloro-N-cyclopentylpyriMidin-4-aMine 2-(Cyanocyclohexyl)acetic acid 4-methoxy-3,5-dinitropyridine 1-(4-(aminomethyl)benzyl)urea hydrochloride 2-aminopropyl benzoate hydrochloride diethyl 2-(2-((tertbutoxycarbonyl)amino) ethyl)malonate tert-butyl 4- (ureidomethyl)benzylcarbamate Ethyl-2-chloro((4-methoxyphenyl)hydrazono)acetateRelated products of tetrahydrofuran


You may like
-
 2033-24-1 98%View Details
2033-24-1 98%View Details
2033-24-1 -
 1975-50-4 98%View Details
1975-50-4 98%View Details
1975-50-4 -
 2-HYDROXY BENZYL ALCOHOL 98%View Details
2-HYDROXY BENZYL ALCOHOL 98%View Details
90-01-7 -
 2-Chloro-1,3-Bis(Dimethylamino)Trimethinium Hexafluorophosphate 221615-75-4 98%View Details
2-Chloro-1,3-Bis(Dimethylamino)Trimethinium Hexafluorophosphate 221615-75-4 98%View Details
221615-75-4 -
 61397-56-6 CIS BROMO BENZOATE 98%View Details
61397-56-6 CIS BROMO BENZOATE 98%View Details
61397-56-6 -
 14714-50-2 (2-Hydroxyphenyl)acetonitrile 98+View Details
14714-50-2 (2-Hydroxyphenyl)acetonitrile 98+View Details
14714-50-2 -
 118753-70-1 98+View Details
118753-70-1 98+View Details
118753-70-1 -
 733039-20-8 5-broMo-2-chloro-N-cyclopentylpyriMidin-4-aMine 98+View Details
733039-20-8 5-broMo-2-chloro-N-cyclopentylpyriMidin-4-aMine 98+View Details
733039-20-8