Bictegravir
- CAS NO.:1611493-60-7
- Empirical Formula: C21H18F3N3O5
- Molecular Weight: 449.38
- MDL number: MFCD31536971
- SAFETY DATA SHEET (SDS)
- Update Date: 2024-11-19 20:33:22
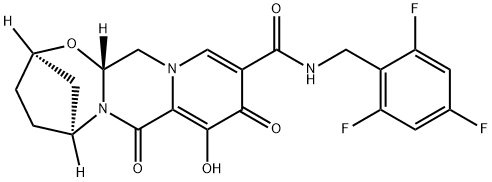
What is Bictegravir?
Absorption
Bictegravir is rapidly absorbed within the body. Tmax= 2.0-4.0h
Toxicity
Those with renal disease and creatinine clearance of <30 mL/min should not take bictegravir .
Patients with hepatic disease should not take bictegravir.
Most common adverse reactions include diarrhea, nausea, and headache .
Lactic acidosis/severe hepatomegaly with steatosis: Discontinue treatment in patients who develop symptoms/laboratory findings suggesting lactic acidosis or hepatotoxicity.
Description
Bictegravir (BIC) is a second-generation integrase strand transfer inhibitor (InSTI), like dolutegravir, with higher genetic barrier to resistance than raltegravir and elvitegravir.
Bictegravir is a human immunodeficiency virus (HIV) integrase strand transfer inhibitor, the fourth in this class of agents that target the viral integrase. Bictegravir is used only in combination with other antiretroviral agents in the treatment of HIV infection and it has had limited use. Bictegravir is associated with a low rate of serum aminotransferase elevations during therapy, but has not been linked to instances of acute, clinically apparent liver injury.
The Uses of Bictegravir
Bictegravir, is a novel, potent inhibitor of HIV-1 integrase with an IC50 of 7.5 nM.
Indications
Bictegravir is indicated in the management of HIV-1 infection in patients not previously treated with antiretroviral therapy. Additionally, Bictegravir is indicated in the management of HIV-1 infection in patients who are virologically suppressed (HIV-1 RNA <50 c/mL) on a regular antiretroviral regimen for a minimum of three months without a history of failure in treatment and no known factors associated with the resistance to the individual components of the medication. It is used in combination with tenofovir and emtricitabine.
Background
Bictegravir is a recently approved investigational drug that has been used in trials studying the treatment of HIV-1 and HIV-2 infection. It has been approved for HIV-1 monotherapy combined with 2 other antiretrovirals in a single tablet.
What are the applications of Application
Bictegravir, is a novel, potent inhibitor of HIV-1 integrase with an IC50 of 7.5 nM. Bictegravir is a recently approved investigational drug that has been used in trials studying the treatment of HIV-1 and HIV-2 infection. It has been approved for HIV-1 monotherapy combined with 2 other antiretrovirals in a single tablet. Bictegravir is indicated in the management of HIV-1 infection in patients not previously treated with antiretroviral therapy. Additionally, Bictegravir is indicated in the management of HIV-1 infection in patients who are virologically suppressed (HIV-1 RNA <50 c/mL) on a regular antiretroviral regimen for a minimum of three months without a history of failure in treatment and no known factors associated with the resistance to the individual components of the medication. It is used in combination with tenofovir and emtricitabine.
Definition
ChEBI: Bictegravir is a monocarboxylic acid amide obtained by formal condensation of the carboxy group of (2R,5S,13aR)-8-hydroxy-7,9-dioxo-2,3,4,5,7,9,13,13a-octahydro-2,5-methanopyrido[1',2':4,5]pyrazino[2,1-b][1,3]oxazepine-10-carboxylic acid with the amino group of 2,4,6-trifluorobenzylamine. It is a second-generation integrase strand transfer inhibitor (INSTI) and used (as its sodium salt) for the treatment of HIV-1. It has a role as a HIV-1 integrase inhibitor. It is a monocarboxylic acid amide, a secondary carboxamide, a trifluorobenzene and an organic heterotetracyclic compound. It is a conjugate acid of a bictegravir(1-).
Mechanism of action
This single dose medication inhibits the strand transfer of viral DNA into the human genome, preventing HIV-1 virus replication and propagation 2.
In vitro, bictegravir has shown powerful antiviral activity against HIV-2 and various subtypes of HIV-1. It has shown synergistic effects when combined with other ARVs, including tenofovir alafenamide (TAF), emtricitabine (FTC), and darunavir (DRV) Label. The three components of the first USA approved medication ( trade name: Biktarvy ) are as follows:
Bictegravir: integrase strand transfer inhibitor; INSTI), an HIV-1 encoded enzyme necessary for viral replication. Inhibition of the integrase enzyme prevents the integration of HIV-1 into host DNA, blocking the conversion of the HIV-1 provirus and progression of the virus.
Emtricitabine: FTC, is phosphorylated by cellular enzymes to form emtricitabine 5'-triphosphate. Emtricitabine is phosphorylated to form emtricitabine 5'-triphosphate intracellularly. This metabolite inhibits the activity of human immunodeficiency virus (HIV) reverse transcriptase by competing with the substrate deoxycytidine 5'-triphosphate and by incorporating itself into viral DNA preventing DNA chain elongation.
Tenofovir Alafenamide: TAF is a phosphonamidate prodrug of tenofovir (2′-deoxyadenosine monophosphate analog). Plasma exposure to TAF leads to leakage into cells and then TAF is intracellularly converted to tenofovir by hydrolysis by cathepsin. Tenofovir is subsequently phosphorylated by cellular kinases to the metabolite tenofovir diphosphate, which is the active form of the drug. Tenofovir diphosphate inhibits HIV-1 replication by incorporating into viral DNA by the HIV reverse transcriptase, resulting in DNA chain-termination. Tenofovir diphosphate also weakly inhibits mammalian DNA polymerases.
Pharmacokinetics
Bictegravir is an HIV-1 integrase strand transfer inhibitor (INSTI). Bictegravir (BIC) inhibits HIV-1 virus replication into the human genome. It can be taken once daily without additional dosing . Bictegravir (BIC) inhibits strand transfer of viral DNA into the host genome and thereby prevents HIV-1 replication .
Pharmacokinetics
Bictegravir is an HIV-1 integrase strand transfer inhibitor (INSTI). Bictegravir (BIC) inhibits HIV-1 virus replication into the human genome. It can be taken once daily without additional dosing. Bictegravir (BIC) inhibits strand transfer of viral DNA into the host genome and thereby prevents HIV-1 replication.
Side Effects
Allergic reactions—skin rash, itching, hives, swelling of the face, lips, tongue, or throat;
High lactic acid level—muscle pain or cramps, stomach pain, trouble breathing, general discomfort and fatigue;
Infection—fever, chills, cough, or sore throat;
Kidney injury—decrease in the amount of urine, swelling of the ankles, hands, or feet;
Liver injury—right upper belly pain, loss of appetite, nausea, light-colored stool, dark yellow or brown urine, yellowing skin or eyes, unusual weakness or fatigue;
Diarrhea, Headache, Nausea
Metabolism
In a 10-day dose-ranging study, monotherapy (5 mg to 100 mg) once daily in adults who were not previously treated with bictegravir, the median half-life of BIC ranged from 15.9 h - 20.9 h . Bictegravir is metabolized in the liver and kidneys. CYP3A4 and UGT1A are the primary enzymes involved in the metabolism of bictegravir. Administration of bictegravir is not advised in patients with renal creatinine clearance of <30 mL/min and patients with hepatic disease .
Properties of Bictegravir
| Melting point: | >130°C (dec.) |
| Boiling point: | 682.5±55.0 °C(Predicted) |
| Density | 1.62±0.1 g/cm3(Predicted) |
| storage temp. | Sealed in dry,Store in freezer, under -20°C |
| solubility | DMSO (Slightly), Methanol (Slightly) |
| form | Solid |
| pka | 4.50±1.00(Predicted) |
| color | White to Off-White |
Safety information for Bictegravir
Computed Descriptors for Bictegravir
Bictegravir manufacturer
Molsyns Research
Shreyaa Medilife Private Limited
New Products
(S)-3-Aminobutanenitrile hydrochloride 4-Methylphenylacetic acid N-Boc-D-alaninol N-BOC-D/L-ALANINOL Tert-butyl bis(2-chloroethyl)carbamate 3-Morpholino-1-(4-nitrophenyl)-5,6-dihydropyridin- 2(1H)-one Furan-2,5-Dicarboxylic Acid Tropic acid 1-Bromo-3,5-Di-Tert-Butylbenzene S-2-CHLORO PROPIONIC ACID ETHYL ISOCYANOACETATE 2-Bromo-1,3-Bis(Dimethylamino)Trimethinium Hexafluorophosphate 4-IODO BENZOIC ACID 3-NITRO-2-METHYL ANILINE 1-(2,4-DICHLOROPHENYL) ETHANAMINE (2-Hydroxyphenyl)acetonitrile 4-Bromopyrazole 2-(Cyanocyclohexyl)acetic acid 4-methoxy-3,5-dinitropyridine 1-(4-(aminomethyl)benzyl)urea hydrochloride 2-aminopropyl benzoate hydrochloride diethyl 2-(2-((tertbutoxycarbonyl)amino) ethyl)malonate tert-butyl 4- (ureidomethyl)benzylcarbamate Ethyl-2-chloro((4-methoxyphenyl)hydrazono)acetateRelated products of tetrahydrofuran
![(4R,12aS)-7-Methoxy-4-Methyl-6,8-dioxo-3,4,6,8,12,12a-hexahydro-2H-[1,3]oxazino[3,2-d]pyrido[1,2-a]pyrazine-9-carboxylic acid](https://img.chemicalbook.in/CAS/20150408/GIF/1335210-34-8.gif)
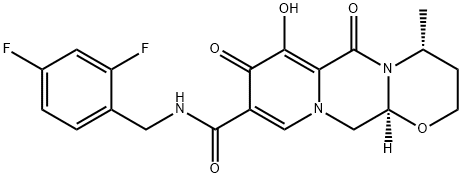
![(4R,12aS)-N-(2,4-Difluorobenzyl)-7-Methoxy-4-Methyl-6,8-dioxo-3,4,6,8,12,12a-hexahydro-2H-[1,3]oxazino[3,2-d]pyrido[1,2-a]pyrazine-9-carboxaMide](https://img.chemicalbook.in/CAS/20150408/GIF/1335210-35-9.gif)
![2-BROMO-1-[4-HYDROXY-3-(HYDROXYMETHYL)PHENYL]ETHAN-1-ONE](https://img.chemicalbook.in/CAS/GIF/62932-94-9.gif)
You may like
-
 Bictegravir 97%View Details
Bictegravir 97%View Details -
 1611493-60-7 98%View Details
1611493-60-7 98%View Details
1611493-60-7 -
 Bictegravir 98%View Details
Bictegravir 98%View Details
1611493-60-7 -
 Bictegravir 1611493-60-7 98%View Details
Bictegravir 1611493-60-7 98%View Details
1611493-60-7 -
 1975-50-4 98%View Details
1975-50-4 98%View Details
1975-50-4 -
 2-HYDROXY BENZYL ALCOHOL 98%View Details
2-HYDROXY BENZYL ALCOHOL 98%View Details
90-01-7 -
 14714-50-2 (2-Hydroxyphenyl)acetonitrile 98+View Details
14714-50-2 (2-Hydroxyphenyl)acetonitrile 98+View Details
14714-50-2 -
 118753-70-1 98+View Details
118753-70-1 98+View Details
118753-70-1