Bestatin
Synonym(s):([2S,3R]-3-Amino-2-hydroxy-4-phenylbutanoyl)-L -leucine;[(2S,3R)-3-Amino-2-hydroxy-4-phenylbutanoyl]-Leu;N-[(2S,3R)-3-Amino-2-hydroxy-4-phenylbutyryl]-L -leucine hydrochloride;Bestatin hydrochloride;N-[(2S,3R)-3-Amino-2-hydroxy-4-phenylbutyryl]-L -leucine hydrochloride
- CAS NO.:58970-76-6
- Empirical Formula: C16H24N2O4
- Molecular Weight: 308.37
- MDL number: MFCD00083262
- EINECS: 261-529-2
- SAFETY DATA SHEET (SDS)
- Update Date: 2025-12-26 16:58:18
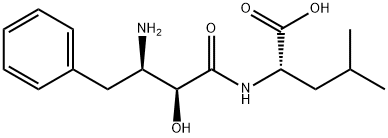
What is Bestatin?
Description
Bestatin is an aminopeptidase inhibitor originally isolated from S. olivoreticuli. It inhibits aminopeptidase B (IC50 = 0.05 μg/ml), aminopeptidase N (IC50 = 16.9 μM), leucine aminopeptidase (IC50 = 0.01 μg/ml), and the aminopeptidase activity of leukotriene A4 (LTA4) hydrolase (Kapp = 172 nM). It is selective for these aminopeptidases over aminopeptidase A, trypsin, chymotrypsin, elastase, papain, pepsin, and thermolysin. Bestatin inhibits the production of LTB4 in erythrocytes when used at a concentration of 70 μM. It increases the expression of Akt, inhibits proliferation, migration, and invasion, and induces autophagy and apoptosis in 5637 bladder cancer cells. Bestatin (5 and 15 mg/kg) decreases serum levels of LTB4 and reduces tumor growth in a patient-derived xenograft (PDX) mouse model of colorectal cancer.
Chemical properties
white powder, soluble in methanol, ethanol, DMSO and other organic solvents, from Streptomyces olivoreticuli.
Originator
Inst. of Microbial Chem (Japan)
The Uses of Bestatin
Ubenimex (also known as bestatin) is a competitive aminopeptidase B inhibitor with an IC50 of 100 mg/ml for K562 cells. Proliferation of all the cell lines except KG1 was inhibited by bestatin. P39/TSU, HL60 and U937 were highly sensitive, with 50% growth inhibitory concentration. It is useful in the treatment of non-lymphocytic leukemia. In other types of cancers ubenimex added to radiotherapy or chemotherapy following surgery significantly inmases survival time through immunopotentiation. It is being studied for use in the treatment of acute myelocytic leukemia.
The Uses of Bestatin
Bestatin is "pseudo"-dipeptide isolated from Strepotmyces olivreticuli in 1976. Bestatin is a potent aminopeptidase B inhibitor with no carboxypeptidase activity. Bestatin acts as an immunomodulator by activating macrophages and T lymphocytes. Bestatin also exhibits good antitumour activity and is has been investigated in clinical trials.
What are the applications of Application
Bestatin is a potent aminopeptidase inhibitor
What are the applications of Application
Bestatin can indirectly act on monocytes by inhibiting Aminopeptidase B, which in turn inhibits the catabolism of tuftsin. Bestatin has also been shown to inhibit Leukotriene A4 hydrolase. It can directly stimulate lymphocytes through its fixation on the cell surface. The agent has been used in multiple leukemia studies. Bestatin is an aminopeptidase inhibitor, inhibits enkephalin metabolism and leukotriene A4 hydrolase. Inhibits tumor cell proliferation.
brand name
Bestatin
General Description
Bestatin was found in the culture broth of Streptomyces olivoreticuli in 1976by Umezawa et al. in the course of screening specific enzyme inhibitors of microbial origin. It shows inhibitory activity against specific exopeptidases, e.g., aminopeptidase B and leucine aminopeptidase. However, it does not act against aminopeptidase A, carboxypeptidases A and B, or endopeptidases. Bestatin acts as a bioresponse modifier and shows antitumor activity via stimulation of immuno response in the host. In combination with cytotoxic anticancer drugs, it is now under clinical evaluation for use in cancer therapy.
Biological Activity
Aminopeptidase inhibitor (K i = 0.001-90 mM); inhibits enkephalin metabolism and leukotriene A 4 hydrolase. Inhibits tumor cell proliferation.
Safety Profile
Poison by intraperitoneal route.Moderately toxic by subcutaneous route. Experimentalreproductive effects. When heated to decomposition itemits toxic fumes of NOx.
Storage
Store at +4°C
Clinical claims and research
Bestatin (ubenimex) is a potent inhibitor of aminopeptidase N and aminopeptidase B, which was isolated from a culture filtrate of Streptomyces olivoreticuli during the search for specific inhibitors of enzymes present on the membrane of eukaryotic cells. Inhibitors of aminopeptidase activity are associated with macrophage activation and differentiation, and Bestatin has shown significant therapeutic effects in several clinical trials. In one multi-institutional study, patients with acute non-lymphocytic leukemia (ANLL) were randomized to receive either Bestatin or control orally after completion of induction and consolidation therapy, and concomitant with maintenance chemotherapy. Remission duration was prolonged in the Bestatin group, although this difference did not reach statistical significance. However, OS was prolonged in the Bestatin group. Recently, a confirmatory phase III trial in ANLL was reported that extended the observation to a significant prolongation of remission. Bestatin has also shown adjuvant activity when administered to acute leukemia and CML patients who did not develop graft-versus-host disease (GVHD) within 30 days following BMT. Bestatin-treated acute leukemia patients had an increased incidence of chronic low-grade GVHD compared with the control arm and a lower relapse rate. Recently, a phase III study of resected stage 1 squamous cell lung cancer patients treated orally with either Bestatin or placebo daily for 2 years revealed that 5-year cancer-free survival was significantly greater in the Bestatin group (71%) as compared to the placebo group (62%). OS was also significantly improved, as was cancer-free survival. Recent studies in patients with nonsmall cell lung cancer (NSCLCs) suggest that it also has anti-angiogenic activity.
Mode of action
Bestatin acts as an immunomodulator by activating macrophages and T lymphocytes.
References
1. umezawa h, aoyagi t, suda h, hamada m, takeuchi t, bestatin, an inhibitor of aminopeptidase b, produced by actinomycetes, j antibiot (tokyo). 1976 jan; 29(1):97-9.2. umezawa, h., aoyagi, t., suda, h., hamada, m., and takeuchi, t. 197615. antibiotics 29, 97.3. suda, h., takita, t., aoyagi, t., and umezawa, h. (1976) j. antibiotics 29, 100.4. nakamura, h., suda, h., takita, t., aoyagi, t., umezawa, h., and iitaka, y. (1976) j. antibiotics 29, 102.5. umezawa, s., tsuchiya, t., and tatsuta, k. (1966) bull. chem. sot. japan 39, 1235. 6. barlow, c. b., and gijthrie, r. d. (1967) j. chem. sot. (c) 1194. 7. bukhari, s. t. k., guthrie, r. d., scott, a. i., and wrixon, a. d. (1970) tetrahedron 26, 3653.8. suda et al. inhibition of aminopeptidase b and leucine aminopeptidase by bestatin and its stereoisomer, archives of biochemistry and biophysics, 77, 196-200 (1976)
Properties of Bestatin
| Melting point: | 245 °C (dec.)(lit.) |
| Boiling point: | 448.76°C (rough estimate) |
| alpha | D20 -15.5° (c = 1.0 in 1N HCl) |
| Density | 1.0917 (rough estimate) |
| refractive index | 1.5800 (estimate) |
| storage temp. | Keep in dark place,Sealed in dry,Room Temperature |
| solubility | Aqueous Acid (Slightly, Sonicated), DMSO (Slightly, Heated), Methanol (Slightly, |
| form | Powder |
| pka | 8.1, 3.1(at 25℃) |
| color | White |
| optical activity | [α]20/D 11°, c = 1 in 1 M NaOH |
| Water Solubility | Soluble in water (<1 25), DMSO (2 mg/ml), methanol (5 mg/ml), 1eq. NaOH (50 mM), ethanol (<1 mg/ml at 25°C), acetic acid, aq. HCl, and alkaline solution. Insoluble in ethyl acetate, benzene, hexane, and chloroform. |
| Merck | 13,9910 |
| CAS DataBase Reference | 58970-76-6(CAS DataBase Reference) |
Safety information for Bestatin
| Signal word | Warning |
| Pictogram(s) |
 Exclamation Mark Irritant GHS07 |
| GHS Hazard Statements |
H315:Skin corrosion/irritation H319:Serious eye damage/eye irritation H335:Specific target organ toxicity, single exposure;Respiratory tract irritation |
| Precautionary Statement Codes |
P271:Use only outdoors or in a well-ventilated area. P280:Wear protective gloves/protective clothing/eye protection/face protection. P312:Call a POISON CENTER or doctor/physician if you feel unwell. P302+P352:IF ON SKIN: wash with plenty of soap and water. P304+P340:IF INHALED: Remove victim to fresh air and Keep at rest in a position comfortable for breathing. P305+P351+P338:IF IN EYES: Rinse cautiously with water for several minutes. Remove contact lenses, if present and easy to do. Continuerinsing. P403+P233:Store in a well-ventilated place. Keep container tightly closed. |
Computed Descriptors for Bestatin
| InChIKey | VGGGPCQERPFHOB-RDBSUJKOSA-N |
New Products
4,4-Difluoropiperidine hydrochloride tert-butyl 9-methoxy-3-azaspiro[5.5]undecane-3-carboxylate Indole Methyl Resin N-Isopropylurea N,N-Dicyclohexylcarbodiimide(DCC) MELDRUMS ACID 5-METHYLISOXAZOLE-4-CARBOXYLIC ACID Magnessium Bis glycinate Zinc ascorbate 1-bromo-2-butyne 2-acetamidophenol 9(10H)-anthracenone Erythrosin B, 4-Piperidinopiperidine 2-((4-morpholinophenylamino) (methylthio) methylene) malononitrile 2,4-dihydroxybenzaldehyde 3-(4-morpholinophenylamino)-5-amino-1H-pyrazole-4-carbonitrile Methyl 2-methylquinoline-6-carboxylate 2,6-dichloro-4-nitropyridine 4-Bromo-2-chlorobenzonitrile 2-(benzylamino)acetic acid hydrochloride 4-(tert-Butoxycarbonylamino)but- 2-ynoic acid 3,4-dihydro-2H-benzo[b][1,4]dioxepine 1-Phenyl-1-cycloprppanecarboxylicacidRelated products of tetrahydrofuran



You may like
-
 Ubenimex 96% CAS 58970-76-6View Details
Ubenimex 96% CAS 58970-76-6View Details
58970-76-6 -
 Bestatin CAS 58970-76-6View Details
Bestatin CAS 58970-76-6View Details
58970-76-6 -
![N-[(2S,3R)-3-Amino-2-hydroxy-4-phenylbutyryl]-L-leucine CAS 58970-76-6](https://img.chemicalbook.in//Content/image/CP5.jpg) N-[(2S,3R)-3-Amino-2-hydroxy-4-phenylbutyryl]-L-leucine CAS 58970-76-6View Details
N-[(2S,3R)-3-Amino-2-hydroxy-4-phenylbutyryl]-L-leucine CAS 58970-76-6View Details
58970-76-6 -
 3-(4-amino-1-oxoisoindolin-2-yl)-1-methylpiperidine-2,6-dione 98%View Details
3-(4-amino-1-oxoisoindolin-2-yl)-1-methylpiperidine-2,6-dione 98%View Details -
 20677-73-0 (2,2-diethoxyethyl)methylamine 98%View Details
20677-73-0 (2,2-diethoxyethyl)methylamine 98%View Details
20677-73-0 -
 3-(4-(hydroxyamino)-1-oxoisoindolin-2-yl)piperidine-2,6-dione 98%View Details
3-(4-(hydroxyamino)-1-oxoisoindolin-2-yl)piperidine-2,6-dione 98%View Details -
 57381-49-4 2-bromo-4-chlorobenzonitrile 98%View Details
57381-49-4 2-bromo-4-chlorobenzonitrile 98%View Details
57381-49-4 -
 4,6-dichloropyrimidine-5-carbaldehyde 98%View Details
4,6-dichloropyrimidine-5-carbaldehyde 98%View Details
5305-40-8
