BERYLLIUM SULFATE
- CAS NO.:13510-49-1
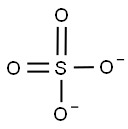
- Empirical Formula: Be.H2O4S
- Molecular Weight: 107.09
- EINECS: 236-842-2
- SAFETY DATA SHEET (SDS)
- Update Date: 2024-12-18 14:08:52
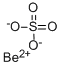
What is BERYLLIUM SULFATE?
Chemical properties
Colorless crystals. Soluble in water; insoluble in alcohol.
Physical properties
Colorless crystalline solid; tetragonal crystal system; hygroscopic; density 2.50 g/cm3 (tetrahydrate 1.71 g/cm3); tetrahydrated salt loses water of crystallization on heating; further heating to 550°C causes decomposition; soluble in water, tetrahydrate more soluble in water (30.5g/100g at 30°) than the anhydrous salt; insoluble in alcohol.
The Uses of BERYLLIUM SULFATE
No major commercial application of beryllium sulfate is known.
The Uses of BERYLLIUM SULFATE
Beryllium sulfate is an important salt of beryllium used as an intermediate of high purity for calcination to beryllium oxide powder for ceramic applications. A saturated aqueous solution of beryllium sulfate contains 30.5% BeSO4 by weight at 30 °C and 65.2% at 111 °C.
Definition
ChEBI: A metal sulfate in which the metal is beryllium (in the +2 oxidation state) and the ratio of beryllium to sulfate is 1:1.
Preparation
Beryllium sulfate may be prepared by treating an aqueous solution of any beryllium salt with sulfuric acid, followed by evaporation of the solution and crystallization. The hydrated product may be converted to anhydrous salt by heating at 400°C.
General Description
Colorless, odorless crystals. Highly toxic by inhalation and ingestion. Sinks and mixes with water.
Air & Water Reactions
Soluble in water.
Reactivity Profile
BERYLLIUM SULFATE is incompatible with the following: Acids, caustics, chlorinated hydrocarbons, oxidizers, molten lithium .
Hazard
A confirmed carcinogen.
Health Hazard
Any dramatic, unexplained weight loss should be considered as possible first indication of beryllium disease. Other symptoms include anorexia, fatigue, weakness, malaise. Inhalation causes pneumonitis, nasopharyngitis, tracheobronchitis, dyspnea, chronic cough. Contact with eyes causes conjunctival inflammation. Contact with skin causes dermatitis of primary irritant or sensitization type; causes ulcer formation when in contact with cuts.
Fire Hazard
Special Hazards of Combustion Products: Toxic beryllium oxide and sulfuric acid fumes may form in fire.
Safety Profile
Confirmed carcinogen withexperimental tumorigenic data. Acute poison byinhalation, ingestion, intraperitoneal, subcutaneous,intravenous, and intratracheal routes. Mutation data reported. When heated to decomposition itemits very toxic fumes of SOx an
Properties of BERYLLIUM SULFATE
| Melting point: | 1127°C |
| Density | 2.500 |
| form | colorless tetragonal crystals |
| color | Colorless, tetragonal, hygroscopic crystals |
| Water Solubility | g/100g solution H2O: 26.69 (0°C), 29.22 (25°C), 45.28 (100°C); solid phase, BeSO4 · 4H2O [KRU93]; insoluble alcohol [HAW93] |
| EPA Substance Registry System | Beryllium sulfate (13510-49-1) |
Safety information for BERYLLIUM SULFATE
Computed Descriptors for BERYLLIUM SULFATE
New Products
(S)-3-Aminobutanenitrile hydrochloride 4-Methylphenylacetic acid N-Boc-D-alaninol N-BOC-D/L-ALANINOL Tert-butyl bis(2-chloroethyl)carbamate 3-Morpholino-1-(4-nitrophenyl)-5,6-dihydropyridin- 2(1H)-one Furan-2,5-Dicarboxylic Acid Tropic acid 1-Bromo-3,5-Di-Tert-Butylbenzene S-2-CHLORO PROPIONIC ACID ETHYL ISOCYANOACETATE 2-Bromo-1,3-Bis(Dimethylamino)Trimethinium Hexafluorophosphate 4-IODO BENZOIC ACID 3-NITRO-2-METHYL ANILINE 1-(2,4-DICHLOROPHENYL) ETHANAMINE (2-Hydroxyphenyl)acetonitrile 4-Bromopyrazole 2-(Cyanocyclohexyl)acetic acid 4-methoxy-3,5-dinitropyridine 1-(4-(aminomethyl)benzyl)urea hydrochloride 2-aminopropyl benzoate hydrochloride diethyl 2-(2-((tertbutoxycarbonyl)amino) ethyl)malonate tert-butyl 4- (ureidomethyl)benzylcarbamate Ethyl-2-chloro((4-methoxyphenyl)hydrazono)acetateRelated products of tetrahydrofuran

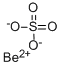

You may like
-
 2033-24-1 98%View Details
2033-24-1 98%View Details
2033-24-1 -
 42831-50-5 5-METHYLISOXAZOLE-4-CARBOXYLIC ACID 98%View Details
42831-50-5 5-METHYLISOXAZOLE-4-CARBOXYLIC ACID 98%View Details
42831-50-5 -
 1975-50-4 98%View Details
1975-50-4 98%View Details
1975-50-4 -
 2-HYDROXY BENZYL ALCOHOL 98%View Details
2-HYDROXY BENZYL ALCOHOL 98%View Details
90-01-7 -
 2-Chloro-1,3-Bis(Dimethylamino)Trimethinium Hexafluorophosphate 221615-75-4 98%View Details
2-Chloro-1,3-Bis(Dimethylamino)Trimethinium Hexafluorophosphate 221615-75-4 98%View Details
221615-75-4 -
 61397-56-6 CIS BROMO BENZOATE 98%View Details
61397-56-6 CIS BROMO BENZOATE 98%View Details
61397-56-6 -
 14714-50-2 (2-Hydroxyphenyl)acetonitrile 98+View Details
14714-50-2 (2-Hydroxyphenyl)acetonitrile 98+View Details
14714-50-2 -
 118753-70-1 98+View Details
118753-70-1 98+View Details
118753-70-1