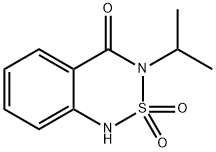
Bentazone
- CAS NO.:25057-89-0
- Empirical Formula: C10H12N2O3S
- Molecular Weight: 240.28
- MDL number: MFCD00078640
- EINECS: 246-585-8
- SAFETY DATA SHEET (SDS)
- Update Date: 2024-12-18 14:08:57
What is Bentazone?
Description
Bentazon is unique under these herbicides because it is the only compound that bears a sulfonyl group. A puzzle that is not solved yet is that bentazone is an excellent herbicide but has a very low pI50-value.
Chemical properties
Bentazon is a colorless to white crystalline powder.
The Uses of Bentazone
Herbicide.
The Uses of Bentazone
Selective, contact, postemergence herbicide used to control a variety of annual and perennial broad-leaved weeds in most grass and legume crops.
Definition
ChEBI: A benzothiadiazine that is 1H-2,1,3-benzothiadiazin-4(3H)-one 2,2-dioxide substituted by an isopropyl group at position 3.
Agricultural Uses
Selective post-emergent herbicide: A post-emergence herbicide used to control broadleaf weeds in crops such as beans, corn, mint, soybeans, rice, and peanuts. All products formerly marketed in the U.S. contain the sodium salt of bentazon as the active ingredient, referred to as sodium bentazon. Also used in selective post-emergent control of broadlelaf weeds and sedges in alfalfa, asparagus, cereals, clover, digitalis, dry peas, flax, garlic, grasses, green lima beans, mint, onions, potatoes, snap beans for seed, sorghum, soybeans and sugarcane. Not currently registered in the U.S. It is reported to be used in most European countries.
Trade name
ASAGIO®; BAS 351-H®; BASAGRAN®; BENDIOXIDE®; BENTA®; BLAST®; ENTRY®; LADDOK®; LEADER®; PLEDGE®; STORM®
Safety Profile
Moderately toxic by ingestion and skin contact. An experimental teratogen. Other experimental reproductive effects. When heated to decomposition it emits very toxic fumes of SO, and NOx.
Potential Exposure
A potential danger to those involved in the manufacture, formulation or application of this selective postemergent thiadiazine herbicide.
First aid
If this chemical gets into the eyes, remove anycontact lenses at once and irrigate immediately for at least15 min, occasionally lifting upper and lower lids. Seekmedical attention immediately. If this chemical contactsthe skin, remove contaminated clothing and wash immediately with soap and water. Seek medical attention immediately. If this chemical has been inhaled, remove fromexposure, begin rescue breathing (using universal precautions, including resuscitation mask) if breathing hasstopped and CPR if heart action has stopped. Transferpromptly to a medical facility. When this chemical hasbeen swallowed, get medical attention. Give large quantities of water and induce vomiting. Do not make an unconscious person vomit.
Environmental Fate
Soil. Under aerobic conditions, bentazone was reported to degrade to 6- and 8-hydrox ybentazone compounds. In addition, anthranilic acid and isopropylamide were reported
as soil hydrolysis products (Otto et al., 1978). Persistence in soil is less than 6 weeks
(Hartley and Kidd, 1987). Bentazone is readily adsorbed onto organic carbon and therefore,
is not expected to leach to groundwater (Abernathy and Wax, 1973). The dissipation half life of bentazone in field soil is 5 days (Ross et al., 1989).
Plant. Undergoes hydroxylation of the aromatic ring and subsequent conju-gation in
plants (Otto et al., 1978; Hartley and Kidd, 1987) forming 6- and 8-hydroxybentazone
compounds (Otto et al., 1978). The half-life in and/or on plants is 2–3 days (
Photolytic. Humburg et al. (1989) reported that 30% degradation of bentazone on glass
plates occurred when exposed to UV light (λ = 200–400 nm); however, no photoproduct(s)
were reported. The natural sunlight and simulated sunlight irradiation of
Chiron et al. (1995) investigated the photodegradation of bentazone (20 μg/L) in
distilled water and Ebro River water using an xenon arc irradiation. In distilled water,
bentazone completely disappeared after 16 hours of irradiation. Photodegradation appeared
to follow pseudo-first-order kinetics with a half-life of about 2.5 hours. The presence of
humic substances (4 mg/L) increased the rate of photodegradation and the disappearance
of bentazone was achieved in 8 hours. No significant breakdown photoproducts were
identified.
Metabolic pathway
14C-Bentazon degrades in soils under conventional tillage and no-tillage (3-18 years) with varying histories of bentazon application. The half-life for bentazon degradation ranges from 4.6 to 49.5 days; half-lives for some no-tillage soils with the longest histories of application are lower than those of conventional tillage soils. Half-lives for soils with no bentazon history are 3-11 times higher than the half- lives of those previously exposed to bentazon. N- Methylbentazon is the most consistently observed metabolite. The other metabolites identified in soils result from hydroxylation on the phenyl ring and the cleavage of the benzothiadiazine ring, yielding 6- and 8-hydroxybentazons and anthranilic acid via 2-amino-N-isopropylbenzamide.
storage
Color Code—Blue: Health Hazard/Poison: Storein a secure poison location. Store in tightly closed containers in a cool, well-ventilated area away from flammablematerials, sources of heat and fire.
Shipping
UN2588 Pesticides, solid, toxic, Hazard Class: 6.1; Labels: 6.1—Poisonous materials, Technical Name Required.
Incompatibilities
Keep away from flammable materials, heat and flame. Risk of fire and explosion if formulations contain flammable/explosive solvents.
Waste Disposal
In accordance with 40CFR165, follow recommendations for the disposal of pesticides and pesticide containers. Must be disposed properly by following package label directions or by contacting your local or federal environmental control agency, or by contacting your regional EPA office.
Properties of Bentazone
| Melting point: | 137-139°C |
| Boiling point: | 395.7±25.0 °C(Predicted) |
| Density | 1.3387 (rough estimate) |
| refractive index | 1.5650 (estimate) |
| Flash point: | 2 °C |
| storage temp. | APPROX 4°C
|
| solubility | Chloroform (Slightly), Methanol (Slightly) |
| form | Solid |
| pka | pKa (24°): 3.3 |
| color | White |
| Water Solubility | 0.5g/L(20 ºC) |
| Merck | 13,1051 |
| BRN | 530220 |
| NIST Chemistry Reference | Bentazone(25057-89-0) |
| EPA Substance Registry System | Bentazon (25057-89-0) |
Safety information for Bentazone
| Signal word | Warning |
| Pictogram(s) |
 Exclamation Mark Irritant GHS07 |
| GHS Hazard Statements |
H302:Acute toxicity,oral H317:Sensitisation, Skin H319:Serious eye damage/eye irritation H412:Hazardous to the aquatic environment, long-term hazard |
| Precautionary Statement Codes |
P261:Avoid breathing dust/fume/gas/mist/vapours/spray. P273:Avoid release to the environment. P280:Wear protective gloves/protective clothing/eye protection/face protection. P301+P312:IF SWALLOWED: call a POISON CENTER or doctor/physician IF you feel unwell. P302+P352:IF ON SKIN: wash with plenty of soap and water. P305+P351+P338:IF IN EYES: Rinse cautiously with water for several minutes. Remove contact lenses, if present and easy to do. Continuerinsing. |
Computed Descriptors for Bentazone
New Products
4-Fluorophenylacetic acid 4-Methylphenylacetic acid N-Boc-D-alaninol N-BOC-D/L-ALANINOL Tert-butyl bis(2-chloroethyl)carbamate 3-Morpholino-1-(4-nitrophenyl)-5,6-dihydropyridin- 2(1H)-one Furan-2,5-Dicarboxylic Acid Tropic acid S-2-CHLORO PROPIONIC ACID ETHYL ISOCYANOACETATE 2-Bromo-1,3-Bis(Dimethylamino)Trimethinium Hexafluorophosphate (6-METHYL-[1,3]DITHIOLO[4,5-b]QUINOXALIN-2-ONE INDAZOLE-3-CARBOXYLIC ACID 4-IODO BENZOIC ACID (2-Hydroxyphenyl)acetonitrile 4-Bromopyrazole 5,6-Dimethoxyindanone 2-(Cyanocyclohexyl)acetic acid 4-methoxy-3,5-dinitropyridine 2-aminopropyl benzoate hydrochloride 1-(4-(aminomethyl)benzyl)urea hydrochloride diethyl 2-(2-((tertbutoxycarbonyl)amino) ethyl)malonate tert-butyl 4- (ureidomethyl)benzylcarbamate Ethyl-2-chloro((4-methoxyphenyl)hydrazono)acetateRelated products of tetrahydrofuran


You may like
-
 Bentazon CAS 25057-89-0View Details
Bentazon CAS 25057-89-0View Details
25057-89-0 -
 Bentazon CAS 25057-89-0View Details
Bentazon CAS 25057-89-0View Details
25057-89-0 -
 Bentazon CAS 25057-89-0View Details
Bentazon CAS 25057-89-0View Details
25057-89-0 -
 1975-50-4 98%View Details
1975-50-4 98%View Details
1975-50-4 -
 2-HYDROXY BENZYL ALCOHOL 98%View Details
2-HYDROXY BENZYL ALCOHOL 98%View Details
90-01-7 -
 2-Chloro-1,3-Bis(Dimethylamino)Trimethinium Hexafluorophosphate 221615-75-4 98%View Details
2-Chloro-1,3-Bis(Dimethylamino)Trimethinium Hexafluorophosphate 221615-75-4 98%View Details
221615-75-4 -
 14714-50-2 (2-Hydroxyphenyl)acetonitrile 98+View Details
14714-50-2 (2-Hydroxyphenyl)acetonitrile 98+View Details
14714-50-2 -
 118753-70-1 98+View Details
118753-70-1 98+View Details
118753-70-1