Bedaquiline fumarate
- CAS NO.:845533-86-0
- Empirical Formula: C36H35BrN2O6
- Molecular Weight: 671.59
- MDL number: MFCD28167761
- SAFETY DATA SHEET (SDS)
- Update Date: 2025-12-16 16:15:04
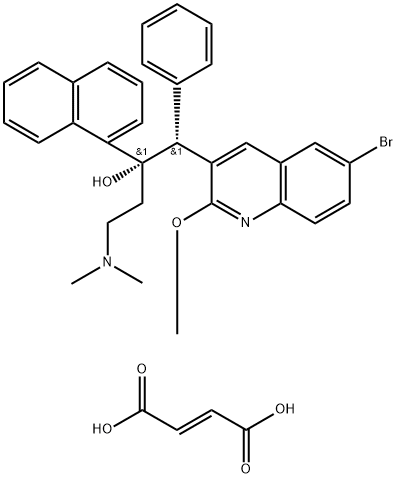
What is Bedaquiline fumarate?
Description
Bedaquiline fumarate (BQF) is an FDA-approved antituberculosis drug that targets the enzyme ATP synthase. It is a fumarate salt prepared from equimolar amounts of bedaquiline and fumaric acid. It can be used in combination therapy for the treatment of multidrug-resistant tuberculosis in the lungs of adults (18 years of age and older). The new BQF microemulsion dosage form of BQF has improved oral bioavailability over the previous formulation, and the BQF microemulsion is cytocompatible with significantly higher cellular uptake than the control group at the highest concentration of 500 μg/ml, which could lead to further use in the effective treatment of multidrug-resistant tuberculosis[1].
Definition
ChEBI: Bedaquiline fumarate is a fumarate salt prepared from equimolar amounts of bedaquiline and fumaric acid. It is used in combination therapy for the treatment of pulmonary multi-drug resistant tuberculosis by inhibition of ATP synthase, an enzyme essential for the replication of the mycobacteria. It has a role as an antitubercular agent and an ATP synthase inhibitor. It contains a bedaquiline(2+).
Clinical Use
Bedaquiline fumarate is a diarylquinone drug developed by Janssen Pharmaceutical which is marketed under the trade name Sirturo ®. The drug, which was approved in 2012 for the treatment of multidrug-resistant tuberculosis (MDR-TB), was developed in partnership with Johnson & Johnson and represents the first new tuberculosis therapy approved in over four decades. Bedaquiline is the first member of a new class of diarylquinone compounds whose mechanism of action inhibits Mycobaterium tuberculosis ATP synthase which deprives bacterium of energy.
Synthesis
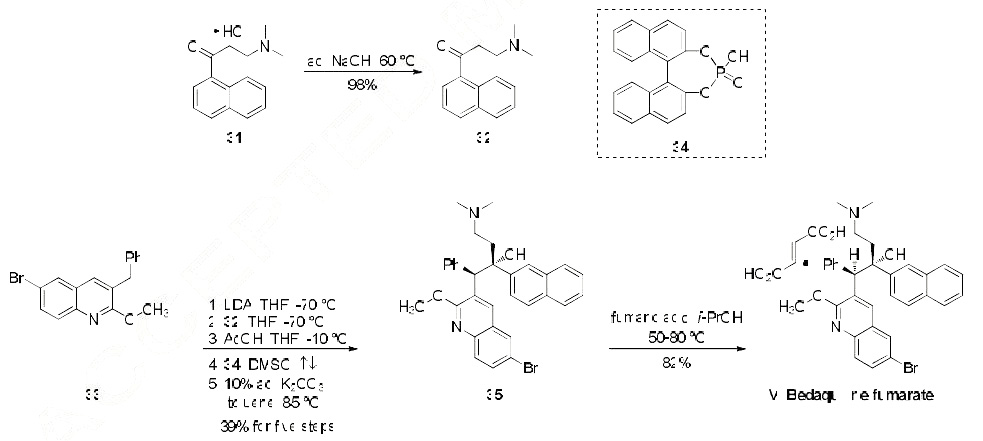
Of the relatively few synthetic approaches to bedaquiline (or its fumarate salt) that have been reported, the most likely process-scale route is that described by Porstmann and co-workers from Janssen Pharmaceutical, and this route is outlined in the scheme. The synthesis was initiated by first freebasing commercially available dimethylaminoketone 31 with sodium hydroxide to provide naphthylone 32 in nearly quantitative yield. Subjection of commercially available quinoline 33 to LDA removed the benzyllic proton within this system and subsequent trap with naphthylone 32 gave rise to a mixture of diastereomers whereby the major diastereomer obtained from this reaction corresponded to the bedaquiline geometry. The minor diastereomer was resolved through multiple recrystallizations and seeding techniques. This racemate of the major diastereomer subsequently underwent a chiral resolution upon treatment with BINAP derivative 34 in refluxing DMSO and then upon cooling and subjection to aqueous base in warm toluene furnished bedaquiline 35 bearing the requisite (R,S)- configuration of the two vicinal chiral centers corresponding to that of the drug. The overall yield of the conversion of 33 to enantiopure 35 was 39%. Aminoquinolinol 35 was then prepared as the corresponding fumarate salt upon treatment with fumaric acid in the presence of isopropanol, and this salt formation delivered bedaquiline fumarate (VI) in 82% yield.
Drug interactions
Potentially hazardous interactions with other drugs
Antibacterials: concentration possibly increased
by ciprofloxacin, clarithromycin and erythromycin
- avoid concomitant use if for more than 14 days;
avoid with moxifloxacin; concentration possibly
reduced by rifampicin - avoid; possibly increased risk
of ventricular arrhythmias with clofazimine.
Antiepileptics: concentration possibly reduced by
carbamazepine, fosphenytoin and phenytoin - avoid.
Antivirals: AUC increased by ritonavir, use with
caution, avoid in combination with lopinavir.
Metabolism
Bedaquiline is metabolised mainly by the hepatic CYP3A4 isoenzyme to the N-monodesmethyl metabolite (M2), which is 4-6 times less active than the parent compound. Bedaquiline is excreted mainly in the faeces.
References
[1] VISHWAS P PARDHI. Bedaquiline fumarate microemulsion: formulation optimization, rheological characterization and in vitro studies.[J]. Nanomedicine (London, England), 2022, 17 21: 1529-1546. DOI:10.2217/nnm-2022-0132.
Properties of Bedaquiline fumarate
| storage temp. | Inert atmosphere,Room Temperature |
| solubility | DMSO : 100 mg/mL (148.90 mM; Need ultrasonic)H2O : < 0.1 mg/mL (insoluble) |
| form | Powder |
| color | White to off-white |
Safety information for Bedaquiline fumarate
| Signal word | Warning |
| Pictogram(s) |
 Exclamation Mark Irritant GHS07 |
| GHS Hazard Statements |
H302:Acute toxicity,oral H315:Skin corrosion/irritation H319:Serious eye damage/eye irritation H335:Specific target organ toxicity, single exposure;Respiratory tract irritation |
| Precautionary Statement Codes |
P261:Avoid breathing dust/fume/gas/mist/vapours/spray. P264:Wash hands thoroughly after handling. P264:Wash skin thouroughly after handling. P270:Do not eat, drink or smoke when using this product. P271:Use only outdoors or in a well-ventilated area. P280:Wear protective gloves/protective clothing/eye protection/face protection. P330:Rinse mouth. P362:Take off contaminated clothing and wash before reuse. P301+P312:IF SWALLOWED: call a POISON CENTER or doctor/physician IF you feel unwell. P302+P352:IF ON SKIN: wash with plenty of soap and water. P304+P340:IF INHALED: Remove victim to fresh air and Keep at rest in a position comfortable for breathing. P305+P351+P338:IF IN EYES: Rinse cautiously with water for several minutes. Remove contact lenses, if present and easy to do. Continuerinsing. P332+P313:IF SKIN irritation occurs: Get medical advice/attention. P337+P313:IF eye irritation persists: Get medical advice/attention. P405:Store locked up. P403+P233:Store in a well-ventilated place. Keep container tightly closed. P501:Dispose of contents/container to..… |
Computed Descriptors for Bedaquiline fumarate
| InChIKey | ZLVSPMRFRHMMOY-KZDJUSSONA-N |
| SMILES | C(/C(=O)O)=C\C(=O)O.[C@](C1=CC=CC2C=CC=CC1=2)(O)(CCN(C)C)[C@H](C1C=CC=CC=1)C1=CC2C=C(Br)C=CC=2N=C1OC |&1:8,25,r| |
New Products
Indole Methyl Resin tert-butyl 9-methoxy-3-azaspiro[5.5]undecane-3-carboxylate Boc-His(Boc)-OH 2-CTC Resin 4-Chloro-7-tosy1-7Hpyrrolo[2,3-d]pyrimidine 5,7-Dibromo-1H-indole 2,5-dichloro-N-hydroxy-4,6-dimethylpyridine-3-carboximidamide 2,2-Dimethoxy-7-azaspiro[3.5]nonane hydrochloride 4-chloromethyl-5-methyl-1,3-dioxol-2-one (DMDO-Cl) R-2-BENZYLOXY PROPIONIC ACID 1,1’-CARBONYLDIIMIDAZOLE 1,1’-CARBONYLDI (1,2-4 TRIAZOLE) N-METHYL INDAZOLE-3-CARBOXYLIC ACID 4-((2-hydroxyethyl)thio)benzoic acid 1-(TERT-BUTOXYCARBONYL)-2-PYRROLIDINONE Methyl 6-methylnicotinate 3-Pyridineacrylic acid tert-Butyl carbazate TETRAHYDRO-2H-PYRAN-3-OL 2-((4-morpholinophenylamino) (methylthio) methylene) malononitrile 3-(4-morpholinophenylamino)-5-amino-1H-pyrazole-4-carbonitrile 2,4-dihydroxybenzaldehyde 1,3-Diethyl-1,3-Diphenylurea Methyl 2-methylquinoline-6-carboxylateRelated products of tetrahydrofuran


You may like
-
 845533-86-0 Bedaquiline Fumarate 98%View Details
845533-86-0 Bedaquiline Fumarate 98%View Details
845533-86-0 -
 845533-86-0 99%View Details
845533-86-0 99%View Details
845533-86-0 -
 Pyridine 99.5% HPLC /UV SpectroscopyView Details
Pyridine 99.5% HPLC /UV SpectroscopyView Details
110-86-1 -
 Piperazine Spot supply, best priceView Details
Piperazine Spot supply, best priceView Details
110-85-0 -
 Dibutyl PhthalateView Details
Dibutyl PhthalateView Details
84-74-2 -
 Imidazole Spot supply, competitive priceView Details
Imidazole Spot supply, competitive priceView Details
288-32-4 -
 Octadecyl 3-(3,5-di-tert-butyl-4-hydroxyphenyl)propionate 98% (GC)View Details
Octadecyl 3-(3,5-di-tert-butyl-4-hydroxyphenyl)propionate 98% (GC)View Details
2082-79-3 -
 Thiourea 99% ARView Details
Thiourea 99% ARView Details
62-56-6
