Lacosamide
- CAS NO.:175481-36-4
- Empirical Formula: C13H18N2O3
- Molecular Weight: 250.29
- MDL number: MFCD08272557
- EINECS: 605-756-0
- SAFETY DATA SHEET (SDS)
- Update Date: 2025-08-21 22:16:43
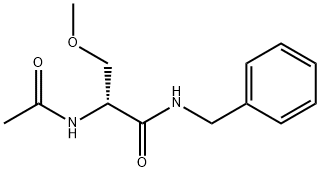
What is Lacosamide?
Absorption
Lacosamide has a negligible first pass effect with bioavailability of about 100%. The maximum Lacosamide plasma concentrations occur about 1-4 hours after oral administration, and the pharmacokinetics of Lacosamide are dose proportional. Food does not affect absorption.
Description
Although epilepsy is a neurological disorder with varying etiology and severity, the common feature is unprovoked, recurring seizures. Whether classified as generalized, involving both cerebral hemispheres, or partial with only localized portions of brain participation at onset, effective treatment relies on accurate assessment of syndrome type to optimally decrease the frequency, duration, and severity of seizures. The latest weapon against partial onset epilepsy is lacosamide, formerly known as harkoseride and erlosamide. The data also indicate that lacosamide binds to collapsing response mediator protein 2 (CRMP2); CRMP2 is involved in neuronal differentiation, control of axonal outgrowth, and possibly epileptogenesis. Furthermore, lacosamide is heralded as having a dual mode of action as it has also displayed efficacy against diabetic neuropathy, possibly as a result of stabilization of neuronal hyperexcitability. Currently,lacosamide is approved as adjunctive treatment of partial onset seizures in patients 17 years or older and is in development as a monotherapy for epilepsy and for neuropathic pain.
Description
Lacosamide selectively enhances sodium channel slow inactivation without affecting fast inactivation. It is effective in multiple rodent models of seizure activity. The neuroprotective effects of lacosamide are also attributed to its ability to modulate collapsin response mediator protein 2 (CRMP-2), a member of the semaphorin signal transduction pathway. Formulations containing lacosamide have been used as an adjunctive or monotherapy for focal-onset seizures but, at higher doses, have a low potential for abuse. Lacosamide is regulated as a Schedule V compound in the United States.
Chemical properties
White to Off-White Solid
Originator
Harris FRC (United States)
The Uses of Lacosamide
A potent anticonvulsant.
Indications
Lacosamide is indicated for the treatment of partial-onset seizures in patients aged one month and older and as adjunctive therapy in the treatment of primary generalized tonic-clonic seizures in patients aged four years and older.
Background
Lacosamide is a functionalized amino acid that has activity in the maximal electroshock seizure test, and is indicated for the adjunctive treatment of partial-onset seizures and diabetic neuropathic pain. Recent studies indicate that Lacosamide only affects those neurons which are depolarized or active for long periods of time, typical of neurons at the focus of an epileptic seizure, as opposed to other antiepileptic drugs such as carbamazepine or lamotrigine which slow the recovery from inactivation and reduce the ability of neurons to fire action potentials.
Definition
ChEBI: Lacosamide is a N-acyl-amino acid.
brand name
Vimpat
Pharmacokinetics
Lacosamide therapy is correlated with a decrease in seizure frequency. It should be noted that in group analyses, dosages above 400 mg/day do not appear to result in additional benefit.
Synthesis
The most common adverse events were diplopia, headache, dizziness, and nausea. As typical with AEDs, lacosamide may increase the risk of suicidal thoughts or behavior. Patients should, therefore, be monitored for the emergence or worsening of depression. Caution should also be exercised in patients with known conduction problems or severe cardiac disease (myocardial ischemia or heart failure) since dose-dependent prolongations in PR interval have been observed in clinical studies.
Metabolism
Lacosamide is a CYP2C19 substrate. The relative contribution of other CYP isoforms or non-CYP enzymes in the metabolism of lacosamide is not known. Primary compounds excreted were unchanged lacosamide (approximately 40% of the dose), its O-desmethyl metabolite (approximately 30%), and a structurally unknown polar fraction (~20%). The plasma exposure of the major human metabolite, O-desmethyl-lacosamide, is approximately 10% of that of lacosamide. This metabolite has no known pharmacological activity.
Properties of Lacosamide
| Melting point: | 141-143?C |
| Boiling point: | 536.4±50.0 °C(Predicted) |
| alpha | D23 +16.0° (c = 1 in CH3OH) |
| Density | 1.120±0.06 g/cm3(Predicted) |
| Flash point: | 2℃ |
| storage temp. | Refrigerator |
| solubility | DMF: 20 mg/ml; DMSO: 20 mg/ml; Ethanol: 20 mg/ml; PBS (pH 7.2): 2 mg/ml |
| form | A crystalline solid |
| pka | 14.19±0.46(Predicted) |
| InChI | InChI=1S/C13H18N2O3/c1-10(16)15-12(9-18-2)13(17)14-8-11-6-4-3-5-7-11/h3-7,12H,8-9H2,1-2H3,(H,14,17)(H,15,16)/t12-/m1/s1 |
| CAS DataBase Reference | 175481-36-4 |
Safety information for Lacosamide
| Signal word | Danger |
| Pictogram(s) |
 Flame Flammables GHS02  Exclamation Mark Irritant GHS07 |
| GHS Hazard Statements |
H225:Flammable liquids H319:Serious eye damage/eye irritation |
| Precautionary Statement Codes |
P210:Keep away from heat/sparks/open flames/hot surfaces. — No smoking. P280:Wear protective gloves/protective clothing/eye protection/face protection. P305+P351+P338:IF IN EYES: Rinse cautiously with water for several minutes. Remove contact lenses, if present and easy to do. Continuerinsing. |
Computed Descriptors for Lacosamide
| InChIKey | VPPJLAIAVCUEMN-GFCCVEGCSA-N |
| SMILES | C(NCC1=CC=CC=C1)(=O)[C@H](NC(C)=O)COC |
Lacosamide manufacturer
Mekanar Pharma Private Limited
New Products
BOC-L-4-HYDROXYPROLINE 2-nitro 3-hydroxy pyridine 2,6-Dichloropyridin-4-amine 2,3 Diamino pyridine 5-Iodo-2-(1-methylethyl)-3(2H)-pyridazinone 1-Azetidinecarboxylic acid, 3-[(3S)-1-(trans-3-carboxy-3-methylcyclobutyl)-3-piperidinyl]-, 1-(1,1-dimethylethyl) ester Tert-Butyl N-[3-(dimethylcarbamoyl)prop-2-en-1-yl]carbamate 1-(difluoromethyl)-N-methylcyclobutan-1-amine 2-(4-Methyl-1,2,5-oxadiazol-3-yl)-1H-benzimidazole 6-(4-iodophenyl)-1-oxa-6-azaspiro[3.3]he Trimethyl(phenylthio)silane Polycaprolactone(2000)-PEG(20000)-Polycaprolactone(2000) Diacrylate Diethylene Glycol Monoethyl Ether, PolyoxyethyleneOleylCetylEtherSulfosuccinate Ascorbyl Tetraisopalmitate or Tetrahexyldecyl Ascorbate Castor Oil, Ethoxylated, Cremophor EL or PEG-35 Castor Oil Tween 20 or Polysorbate 20 Acetone-d6 (R)-2-Mercaptobutanoic acid 3-iodo-1H-pyrazolo[3,4-d]pyrimidin-4-amine 3-(naphthalen-1-ylsulfonyl)-1H-indazol-5-amine methyl 5-amino-3-(1,1-dioxidotetrahydro-2H-1,2-thiazin-2-yl)-2-fluorobenzoate 7-methoxy-8-(2-morpholinoethoxy)-4-((3,4,5-trimethoxyphenyl)amino)benzo[g]quinoline-3-carbonitrile Dimethylaluminum isopropoxideRelated products of tetrahydrofuran






You may like
-
 175481-36-4 98%View Details
175481-36-4 98%View Details
175481-36-4 -
 S-Lacosamide 175481-36-4 98%View Details
S-Lacosamide 175481-36-4 98%View Details
175481-36-4 -
 Lacosamide 98%View Details
Lacosamide 98%View Details -
 Lacosamide 175481-36-4 98%View Details
Lacosamide 175481-36-4 98%View Details
175481-36-4 -
 Lacosamide 98%View Details
Lacosamide 98%View Details -
 175481-36-4 98%View Details
175481-36-4 98%View Details
175481-36-4 -
 LACOSAMIDE 95-99 %View Details
LACOSAMIDE 95-99 %View Details
175481-36-4 -
 LACOSAMIDE 99%View Details
LACOSAMIDE 99%View Details
