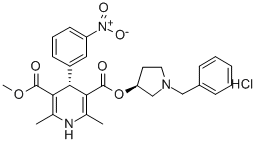
Barnidipine hydrochloride
- CAS NO.:104757-53-1
- Empirical Formula: C27H30ClN3O6
- Molecular Weight: 528
- MDL number: MFCD00915772
- SAFETY DATA SHEET (SDS)
- Update Date: 2024-11-17 19:25:39
What is Barnidipine hydrochloride?
Description
Barnidipine hydrochloride is a vasodilating dihydropyridine calcium antagonist useful in the treatment of hypertension. In experimental animals, its hypotensive effect was more potent and longer acting than nicardipine, nifedipine and nitrendipine.
Description
Barnidipine hydrochloride, chemically known as (3S)-1-benzyl-3-pyrrolidinylmethyl-(4S)-2,6-dimethyl-4-(m-nitrophenyl)-1,4-dihydropyridine-3,5-dicarboxylate hydrochloride, is a new long-acting dihydropyridine calcium antagonist developed and marketed by Yamanouchi Pharmaceutical (now Astellas Pharma). This medication effectively lowers blood pressure without impacting heart rate by specifically targeting calcium channels responsible for transmembrane potential. By inhibiting calcium influx into cells, it selectively relaxes smooth muscle in peripheral and coronary blood vessels.
Chemical properties
Pale Yellow Solid. Melting point 226-228°C. [α]D20+116.4° (C=1, methanol). Insoluble in water. Acute toxicity LD50 male and female rats (mg/kg): 105, 113 Oral.
Originator
Yamanouchi (Japan)
The Uses of Barnidipine hydrochloride
1,4-Dihydropyridine calcium antagonist with antihypertensive and diuretic effects. A potential candidate in the treatment of patients with renal parenchymal hypertension.
Definition
ChEBI: Barnidipine hydrochloride is a dihydropyridine. 1,4-Dihydropyridine calcium antagonist with antihypertensive and diuretic effects. It is a potential candidate in the treatment of patients with renal parenchymal hypertension.
Manufacturing Process
In 5 ml of isopropyl alcohol were dissolved 1.5 g (0.01 mole) of 3- nitrobenzaldehyde, 2.6 g (0.01 mole) of 1-benzyl-3-acetoacetyloxypyrrolidine, and 1.3 g (0.01 mole) of β-aminocrotonic acid methyl ester and then the solution was refluxed for 8 hours. The solvent was distilled off under reduced pressure, the residue obtained was dissolved in a small amount of chloroform, and the solution was applied to silica gel column chromatography (column diameter 1.5 cm, height 20 cm, and about 200 ml of chloroform was used as the eluent). The eluates were collected and concentrated to give 3.4 g of oily 2,6-dimethyl-4-(3'-nitrophenyl)-1,4-dihydropyridine-3,5-dicarboxylic acid-3- (1-benzylpyrrolidin-3-yl)ester-5-methyl ester: [α]D 20=+64.8° (c = 1 in methanol).
brand name
Hypoca
Therapeutic Function
Coronary vasodilator
Biological Activity
Barnidipine is a dihydropyridine calcium channel blocker that has an IC50 value of 0.35 nM in potassium-induced tonic contraction of pig coronary artery. It demonstrates antihypertensive activity by reducing peripheral vascular resistance. It decreases blood pressure in spontaneously hypertensive rats when administered orally at 1 and 3 mg/kg per day. Formulations containing barnidipine have been used as a treatment for hypertension.
in vitro
the effects of barnidipine on l-type ca(2+) current (i(ca(l))) were investigated in rat ventricular cardiomyocytes. it was found that barnidipine reduced i(ca(l)) in a concentration and voltage dependent manne. barnidipine induced a leftward shift of the steady-state inactivation curve of i(ca(l)) [1].
in vivo
a previous study was conducted to investigate the influence of barnidipine treatment on early stage hypertension by determining the mesenteric and renal arteries as well as the kidney in l-name-induced hypertensive rats. barnidipine was applied to rats after 2 weeks of l-name administration, and continued for the next 3 weeks concomitantly with l-name. histopathological studies verified structural alterations in the arteries and the kidney. moreover, a decrease in endothelial nitric oxide synthase expression was observed both in the arteries and kidney of hypertensive rats with barnidipine treatment [2].
References
[1] WEGENERJ W. Barnidipine block of L-type Ca(2+) channel currents in rat ventricular cardiomyocytes.[J]. British Journal of Pharmacology, 2000. DOI:10.1038/sj.bjp.0703514.
[2] ALP YILDIRIMF ILKAY. Barnidipine ameliorates the vascular and renal injury in L-NAME-induced hypertensive rats.[J]. European journal of pharmacology, 2015. DOI:10.1016/j.ejphar.2015.07.033.
[3] CHENGZHI-GANG. Synthesis and characterization of impurities of barnidipine hydrochloride, an antihypertensive drug substance.[J]. Molecules, 2014. DOI:10.3390/molecules19011344.
Properties of Barnidipine hydrochloride
| Melting point: | 223-226°C |
| alpha | D20 +116.4° (c = 1 in methanol) |
| storage temp. | Sealed in dry,Room Temperature |
| solubility | insoluble in H2O; insoluble in EtOH; ≥16.3 mg/mL in DMSO with gentle warming |
| form | A crystalline solid |
| color | Light yellow to yellow |
| CAS DataBase Reference | 104757-53-1(CAS DataBase Reference) |
Safety information for Barnidipine hydrochloride
| Signal word | Warning |
| Pictogram(s) |
 Exclamation Mark Irritant GHS07 |
| GHS Hazard Statements |
H317:Sensitisation, Skin H319:Serious eye damage/eye irritation |
| Precautionary Statement Codes |
P280:Wear protective gloves/protective clothing/eye protection/face protection. P305+P351+P338:IF IN EYES: Rinse cautiously with water for several minutes. Remove contact lenses, if present and easy to do. Continuerinsing. |
Computed Descriptors for Barnidipine hydrochloride
New Products
4-Fluorophenylacetic acid 4-Methylphenylacetic acid N-Boc-D-alaninol N-BOC-D/L-ALANINOL Tert-butyl bis(2-chloroethyl)carbamate 3-Morpholino-1-(4-nitrophenyl)-5,6-dihydropyridin- 2(1H)-one Furan-2,5-Dicarboxylic Acid Tropic acid S-2-CHLORO PROPIONIC ACID ETHYL ISOCYANOACETATE 2-Bromo-1,3-Bis(Dimethylamino)Trimethinium Hexafluorophosphate (6-METHYL-[1,3]DITHIOLO[4,5-b]QUINOXALIN-2-ONE INDAZOLE-3-CARBOXYLIC ACID 4-IODO BENZOIC ACID (2-Hydroxyphenyl)acetonitrile 4-Bromopyrazole 5,6-Dimethoxyindanone 2-(Cyanocyclohexyl)acetic acid 4-methoxy-3,5-dinitropyridine 2-aminopropyl benzoate hydrochloride 1-(4-(aminomethyl)benzyl)urea hydrochloride diethyl 2-(2-((tertbutoxycarbonyl)amino) ethyl)malonate tert-butyl 4- (ureidomethyl)benzylcarbamate Ethyl-2-chloro((4-methoxyphenyl)hydrazono)acetateRelated products of tetrahydrofuran



You may like
-
 Barnidipine HCl 98% (HPLC) CAS 104757-53-1View Details
Barnidipine HCl 98% (HPLC) CAS 104757-53-1View Details
104757-53-1 -
 2033-24-1 98%View Details
2033-24-1 98%View Details
2033-24-1 -
 1975-50-4 98%View Details
1975-50-4 98%View Details
1975-50-4 -
 2-HYDROXY BENZYL ALCOHOL 98%View Details
2-HYDROXY BENZYL ALCOHOL 98%View Details
90-01-7 -
 2-Chloro-1,3-Bis(Dimethylamino)Trimethinium Hexafluorophosphate 221615-75-4 98%View Details
2-Chloro-1,3-Bis(Dimethylamino)Trimethinium Hexafluorophosphate 221615-75-4 98%View Details
221615-75-4 -
 61397-56-6 CIS BROMO BENZOATE 98%View Details
61397-56-6 CIS BROMO BENZOATE 98%View Details
61397-56-6 -
 14714-50-2 (2-Hydroxyphenyl)acetonitrile 98+View Details
14714-50-2 (2-Hydroxyphenyl)acetonitrile 98+View Details
14714-50-2 -
 118753-70-1 98+View Details
118753-70-1 98+View Details
118753-70-1