BARIUM SELENATE
- CAS NO.:7787-41-9
- Empirical Formula: BaH4O4Se
- Molecular Weight: 284.33
- MDL number: MFCD00046181
- EINECS: 232-113-8
- SAFETY DATA SHEET (SDS)
- Update Date: 2023-10-17 17:11:42
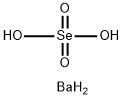
What is BARIUM SELENATE?
Description
Barium selanate has the molecular formula of BaSeO4
and the molecular weight of 280.2748 g/mol. It can be
prepared by reacting sodium selenate with barium chloride
in solution:
BaCl2(aq)+ Na2SeO4(aq) ? BaSeO4+ 2NaCl(aq)
Barium selenate is slightly soluble in water at
0.0052 g/100 ml at 20°C. Its CAS number is 7787-41-9.
It is a white rhombic crystal which decomposes when
heated beyond about 425°C. It is slightly soluble in
water at 0.0152 g/100 ml at 20°C. Its solubility product
is 3.40×10-8.
The heat of formation of barium selenate is:
△H298 BaSeO4 = -249.0 kcal/mol (-1041.8 kJ/mol).
As a result of the measurements, it has been found
that for the reaction:
Basolid+ Sesolid+ 2O2 = BaSeO4
△H298 = -279.2 cal/mol
△F298 = -249.1 cal/mol
Chemical properties
orthorhombic crystal(s); preparation: heating BaCO3 and Se [MER06]
Reactivity Profile
Inorganic oxidizing agents can react with reducing agents to generate heat and products that may be gaseous (causing pressurization of closed containers). The products may themselves be capable of further reactions (such as combustion in the air). The chemical reduction of materials in this group can be rapid or even explosive, but often requires initiation (heat, spark, catalyst, addition of a solvent). Explosive mixtures of inorganic oxidizing agents with reducing agents often persist unchanged for long periods if initiation is prevented. Such systems are typically mixtures of solids, but may involve any combination of physical states. Some inorganic oxidizing agents are salts of metals that are soluble in water; dissolution dilutes but does not nullify the oxidizing power of such materials. Organic compounds in general have some reducing power and can in principle react with compounds in this class. Actual reactivity varies greatly with the identity of the organic compound. Inorganic oxidizing agents can react violently with active metals, cyanides, esters, and thiocyanates. Explosives often consist of an inorganic oxidizing agent mixed in intimate contact with a reducing agent. Gunpowder is such a mixture. Other examples are a mixture of sugar (an organic compound) plus sodium chlorate and magnesium (an inorganic reducing agent) plus barium peroxide.
Health Hazard
Highly toxic, may be fatal if inhaled, swallowed or absorbed through skin. Avoid any skin contact. Effects of contact or inhalation may be delayed. Fire may produce irritating, corrosive and/or toxic gases. Runoff from fire control or dilution water may be corrosive and/or toxic and cause pollution.
Fire Hazard
Non-combustible, substance itself does not burn but may decompose upon heating to produce corrosive and/or toxic fumes. Containers may explode when heated. Runoff may pollute waterways.
Properties of BARIUM SELENATE
| Melting point: | >350 °C(lit.) |
| Density | 4.750 |
| form | white rhombohedral crystals |
| color | white rhomb crystals, crystalline |
| Water Solubility | g/L soln; H2O; 0.081 (25°C); soluble HCl, insoluble HNO3 [MER06] [KRU93] |
| Solubility Product Constant (Ksp) | pKsp: 7.47 |
| Exposure limits | ACGIH: TWA 0.5 mg/m3; TWA 0.2 mg/m3 NIOSH: IDLH 50 mg/m3; IDLH 1 mg/m3; TWA 0.5 mg/m3; TWA 0.2 mg/m3 |
| CAS DataBase Reference | 7787-41-9 |
| EPA Substance Registry System | Selenic acid, barium salt (1:1) (7787-41-9) |
Safety information for BARIUM SELENATE
| Pictogram(s) |
 Skull and Crossbones Acute Toxicity GHS06  Health Hazard GHS08  Environment GHS09 |
| GHS Hazard Statements |
H301:Acute toxicity,oral H331:Acute toxicity,inhalation H373:Specific target organ toxicity, repeated exposure H400:Hazardous to the aquatic environment, acute hazard H410:Hazardous to the aquatic environment, long-term hazard |
| Precautionary Statement Codes |
P260:Do not breathe dust/fume/gas/mist/vapours/spray. P304+P340:IF INHALED: Remove victim to fresh air and Keep at rest in a position comfortable for breathing. P405:Store locked up. |
Computed Descriptors for BARIUM SELENATE
New Products
4-AMINO-TETRAHYDRO-PYRAN-4-CARBOXYLIC ACID HCL 4-(Dimethylamino)tetrahydro-2H-pyran-4-carbonitrile 4-Aminotetrahydropyran-4-carbonitrile Hydrochloride (R)-3-Aminobutanenitrile Hydrochloride 3-((Dimethylamino)methyl)-5-methylhexan-2-one oxalate 1,4-Dioxa-8-azaspiro[4.5]decane 5-Bromo-2-nitropyridine Nimesulide BP Aceclofenac IP/BP/EP Diclofenac Sodium IP/BP/EP/USP Mefenamic Acid IP/BP/EP/USP Ornidazole IP Diclofenac Potassium THOMAIND PAPER PH 2.0 TO 4.5 1 BOX BUFFER CAPSULE PH 9.2 - 10 CAP SODIUM CHLORIDE 0.1N CVS ALLOXAN MONOHYDRATE 98% PLATINUM 0.5% ON 3 MM ALUMINA PELLETS (TYPE 73) LITHIUM AAS SOLUTION 2-Bromo-1-(bromomethyl)-3-chloro-5-nitrobenzene 2-Bromo-3-nitroaniline N-(3-Hydroxypropyl)-N-methylacetamide 3-Bromo-6-chloropyridazine 4-ethyl-3-nitrobenzoic acidRelated products of tetrahydrofuran

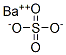
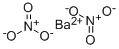
You may like
-
 1-Methyl-6-oxo-1,6-dihydropyridazine-3-carbonitrile 98%View Details
1-Methyl-6-oxo-1,6-dihydropyridazine-3-carbonitrile 98%View Details
99903-60-3 -
 88491-46-7 98%View Details
88491-46-7 98%View Details
88491-46-7 -
 1823368-42-8 98%View Details
1823368-42-8 98%View Details
1823368-42-8 -
 2-(3-(tert-butyl)phenoxy)-2-methylpropanoic acid 1307449-08-6 98%View Details
2-(3-(tert-butyl)phenoxy)-2-methylpropanoic acid 1307449-08-6 98%View Details
1307449-08-6 -
 Ethyl 3-(furan-2-yl)-3-hydroxypropanoate 25408-95-1 98%View Details
Ethyl 3-(furan-2-yl)-3-hydroxypropanoate 25408-95-1 98%View Details
25408-95-1 -
 2-Chloro-5-fluoro-1-methoxy-3-methylbenzene 98%View Details
2-Chloro-5-fluoro-1-methoxy-3-methylbenzene 98%View Details
1805639-70-6 -
 1784294-80-9 98%View Details
1784294-80-9 98%View Details
1784294-80-9 -
 Lithium ClavulanateView Details
Lithium ClavulanateView Details
61177-44-4