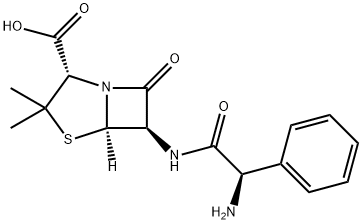
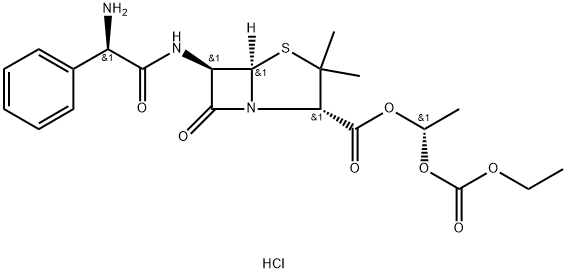
BACAMPICILLIN
- CAS NO.:50972-17-3
- Empirical Formula: C21H27N3O7S
- Molecular Weight: 465.52
- MDL number: MFCD00864977
- SAFETY DATA SHEET (SDS)
- Update Date: 2023-05-21 10:59:17
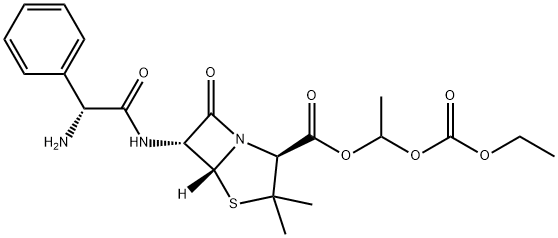
What is BACAMPICILLIN?
Absorption
Absorbed following oral administration.
Originator
Penglobe,Astra,W. Germany,1977
Background
Bacampicillin is a prodrug of ampicillin and is microbiologically inactive. It is absorbed following oral administration. During absorption from the gastrointestinal tract, bacampicillin is hydrolyzed by esterases present in the intestinal wall. It is microbiologically active as ampicillin, and exerts a bactericidal action through the inhibition of the biosynthesis of cell wall mucopeptides. It is used to cure infection of upper and lower respiratory tract; skin and soft tissue; urinary tract and acute uncomplicated gonococcal urethritis etc.
Indications
For infections at the following sites: upper and lower respiratory tract; skin and soft tissue; urinary tract and acute uncomplicated gonococcal urethritis, when due to sensitive strains of the following organisms: Gram-positive: streptococci (including S. faecalis and S. pneumoniae) and nonpenicillinase-producing staphylococci; Gram-negative: H. influenzae, N. gonorrhoeae, E. coli, P. mirabilis, Salmonellae and Shigellae.
Definition
ChEBI: A penicillanic acid ester that is the 1-ethoxycarbonyloxyethyl ester of ampicillin. It is a semi-synthetic, microbiologically inactive prodrug of ampicillin.
Manufacturing Process
1'-Ethoxycarbonyloxyethyl 6-(D-α-azidophenylacetamido)penicillinate (98 g)
was prepared from sodium 6-(D-α-azidophenylacetamido)penicillinate (397 g,
1 mol), α-chlorodiethylcarbonate (458 g, 3 mols) and sodium bicarbonate
(504 g, 6 mols). The product showed strong IR absorption at 2090 cm-1 and
1780-1750 cm-1 showing the presence of azido group and β-lactam and ester
carbonyls.
It was dissolved in ethyl acetate (700 ml) and hydrogenated at ambient
conditions over a palladium (5%)on carbon catalyst (18 g). The catalyst was
removed by filtration and washed with ethyl acetate. The combined filtrates
were extracted with water at pH 2.5 by addition of dilute hydrochloric acid.
Lyophilization of the aqueous phase gave the hydrochloride of 1'-
ethoxycarbonyloxyethyl 6-(D-α-aminophenylacetarnido)penicillinate (94 g), MP
171°-176°C.
Therapeutic Function
Antibacterial
Pharmacokinetics
Bacampicillin is a prodrug of ampicillin and is microbiologically inactive.
Metabolism
Not Available
Metabolism
Although comparatively well absorbed (30–55%), the oral efficacy of ampicillin for systemic infections can be enhanced significantly through the preparation of pro-drugs. In contrast to ampicillin itself, which is amphoteric, bacampicillin is a weak base and is very well absorbed in the duodenum (80–98%). Enzymatic ester hydrolysis in the gut wall liberates carbon dioxide and ethanol, followed by spontaneous loss of acetaldehyde and production of ampicillin. The acetaldehyde is metabolized oxidatively by alcohol dehydrogenase to produce acetic acid, which joins the normal metabolic pool.
Properties of BACAMPICILLIN
| Boiling point: | 678.4±55.0 °C(Predicted) |
| Density | 1.37±0.1 g/cm3(Predicted) |
| storage temp. | Store at -20°C |
| solubility | Soluble in DMSO |
| pka | pKa 6.8 (Uncertain) |
| CAS DataBase Reference | 50972-17-3 |
Safety information for BACAMPICILLIN
Computed Descriptors for BACAMPICILLIN
New Products
4-Fluorophenylacetic acid 4-Methylphenylacetic acid N-Boc-D-alaninol N-BOC-D/L-ALANINOL Tert-butyl bis(2-chloroethyl)carbamate 3-Morpholino-1-(4-nitrophenyl)-5,6-dihydropyridin- 2(1H)-one Furan-2,5-Dicarboxylic Acid Tropic acid S-2-CHLORO PROPIONIC ACID ETHYL ISOCYANOACETATE 2-Bromo-1,3-Bis(Dimethylamino)Trimethinium Hexafluorophosphate (6-METHYL-[1,3]DITHIOLO[4,5-b]QUINOXALIN-2-ONE INDAZOLE-3-CARBOXYLIC ACID 4-IODO BENZOIC ACID (2-Hydroxyphenyl)acetonitrile 4-Bromopyrazole 5,6-Dimethoxyindanone 2-(Cyanocyclohexyl)acetic acid 4-methoxy-3,5-dinitropyridine 2-aminopropyl benzoate hydrochloride 1-(4-(aminomethyl)benzyl)urea hydrochloride diethyl 2-(2-((tertbutoxycarbonyl)amino) ethyl)malonate tert-butyl 4- (ureidomethyl)benzylcarbamate Ethyl-2-chloro((4-methoxyphenyl)hydrazono)acetateRelated products of tetrahydrofuran


You may like
-
 2033-24-1 98%View Details
2033-24-1 98%View Details
2033-24-1 -
 42831-50-5 5-METHYLISOXAZOLE-4-CARBOXYLIC ACID 98%View Details
42831-50-5 5-METHYLISOXAZOLE-4-CARBOXYLIC ACID 98%View Details
42831-50-5 -
 1975-50-4 98%View Details
1975-50-4 98%View Details
1975-50-4 -
 2-HYDROXY BENZYL ALCOHOL 98%View Details
2-HYDROXY BENZYL ALCOHOL 98%View Details
90-01-7 -
 2-Chloro-1,3-Bis(Dimethylamino)Trimethinium Hexafluorophosphate 221615-75-4 98%View Details
2-Chloro-1,3-Bis(Dimethylamino)Trimethinium Hexafluorophosphate 221615-75-4 98%View Details
221615-75-4 -
 61397-56-6 CIS BROMO BENZOATE 98%View Details
61397-56-6 CIS BROMO BENZOATE 98%View Details
61397-56-6 -
 14714-50-2 (2-Hydroxyphenyl)acetonitrile 98+View Details
14714-50-2 (2-Hydroxyphenyl)acetonitrile 98+View Details
14714-50-2 -
 118753-70-1 98+View Details
118753-70-1 98+View Details
118753-70-1