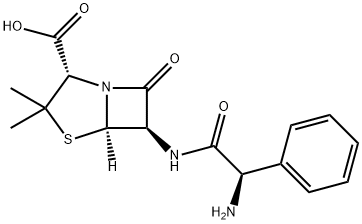
PIVAMPICILLIN
- CAS NO.:33817-20-8
- Empirical Formula: C22H29N3O6S
- Molecular Weight: 463.55
- MDL number: MFCD00869402
- EINECS: 251-688-6
- SAFETY DATA SHEET (SDS)
- Update Date: 2023-05-04 17:34:37
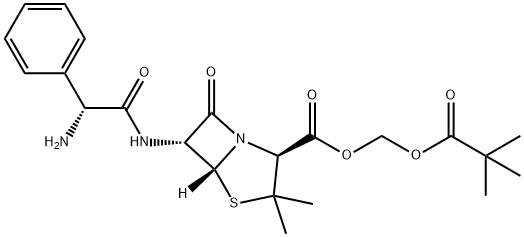
What is PIVAMPICILLIN?
Absorption
Absorbed following oral administration.
Description
Pivampicillin caused sensitization in 56 workers at a penicillin factory. Pivampicillin and pivmecillinam were responsible for contact dermatitis in pharmaceutical production workers. Ampicillin, mecillinam, penicillin V and penicillin G were also implicated in cross reactions.
Chemical properties
White or almost white, crystalline powder.
Originator
Maxifen ,Sharp and Dohme, W. Germany ,1972
The Uses of PIVAMPICILLIN
Antibacterial.
Indications
or the treatment of respiratory tract infections (including acute bronchitis, acute exacerbations of chronic bronchitis and pneumonia); ear, nose and throat infections; gynecological infections; urinary tract infections (including acute uncomplicated gonococcal urethritis) when caused by non penicillinase-producing susceptible strains of the following organisms: gram-positive organisms, e.g., streptococci, pneumococci and staphylococci; gram-negative organisms, e.g., H. influenzae, N. gonorrhoeae, E. coli, P. mirabilis.
Background
Pivalate ester analog of ampicillin.
Definition
ChEBI: Pivampicillin is a penicillanic acid ester that is the pivaloyloxymethyl ester of ampicillin. It is a prodrug of ampicillin. It has a role as a prodrug. It is a penicillanic acid ester and a pivaloyloxymethyl ester. It is functionally related to an ampicillin.
Manufacturing Process
(A) Pivaloyloxymethyl D(-)-α-azidobenzylpenicillinate: To a suspension of potassium D(-)α-azidobenzylpenicillinate (4.14 g) and potassium dicarbonate(1.5 g) in acetone (100 ml) and 10% aqueous sodium iodide (2 ml), chloromethyl pivalate (2.7 ml) was added and the mixture refluxed for 2 hours. After cooling, the suspension was filtered and the filtrate evaporated to dryness in vacuo. The remaining residue was washed repeatedly by decantation with petroleum ether to remove unreacted chloromethyl pivalate. The oily residue was taken up in ethyl acetate (100 ml), and the resulting solution washed with aqueous sodium bicarbonate and water, dried and evaporated in vacuo to yield the desired compound as a yellowish gum, which crystallized from ether, melting point 114°C to 115°C.
(B) Pivaloyloxymethyl D(-)-α-aminobenzylpenicillinate, hydrochloride: To a solution of pivaloyloxymethyl D(-)-α-azidobenzylpenicillinate (prepared as described above) in ethyl acetate (75 ml) a 0.2 M phosphate buffer (pH 2.2) (75 ml) and 10% palladium on carbon catalyst (4 g) were added, and the mixture was shaken in a hydrogen atmosphere for 2 hours at room temperature. The catalyst was filtered off, washed with ethyl acetate (25 ml) and phosphate buffer (25 ml), and the phases of the filtrate were separated. The aqueous phase was washed with ether, neutralized (pH 6.5 to 7.0) with aqueous sodium bicarbonate, and extracted with ethyl acetate (2 x 75 ml). To the combined extracts, water (75 ml) was added, and the pH adjusted to 2.5 with 1 N hydrochloric acid. The aqueous layer was separated, the organic phase extracted with water (25 ml), and the combined extracts were washed with ether, and freeze-dried. The desired compound was obtained as a colorless, amorphous powder.
The purity of the compound was determined iodometrically to be 91%. A crystalline hydrochloride was obtained from isopropanol with a melting point of 155°C to 156°C (dec.).
Therapeutic Function
Antibacterial
Contact allergens
Pivampicillin is a prodrug of ampicillin. It caused sensitization in 56 workers at a penicillin factory. Pivampicillin and pivmecillinam were responsible for contact dermatitis in pharmaceutical production workers. Ampicillin, mecillinam or amdinocillin, penicillin V and penicillin G were also implicated in cross-reactions.
Pharmacokinetics
Pivampicillin is the pivaloyloxymethyl ester of (the semi-synthetic penicillin) ampicillin. It is an inactive pro-drug, which is converted during its absorption from the gastrointestinal tract to the microbiologically active ampicillin, together with formaldehyde and pivalic acid, by non-specific esterases present in most body tissues. Amounts in excess of 99% of the pivampicillin absorbed are converted to ampicillin within 15 minutes of absorption.
Metabolism
Not Available
Properties of PIVAMPICILLIN
| Boiling point: | 679.0±55.0 °C(Predicted) |
| Density | 1.33±0.1 g/cm3(Predicted) |
| solubility | Practically insoluble in water, freely soluble in methanol, soluble in anhydrous ethanol. It dissolves in dilute acids. |
| pka | pKa 7.0 (Uncertain) |
Safety information for PIVAMPICILLIN
Computed Descriptors for PIVAMPICILLIN
New Products
Tert-butyl bis(2-chloroethyl)carbamate (S)-3-Aminobutanenitrile hydrochloride N-Boc-D-alaninol N-BOC-D/L-ALANINOL 3-Morpholino-1-(4-nitrophenyl)-5,6-dihydropyridin- 2(1H)-one N-octanoyl benzotriazole 3,4-Dibenzyloxybenzaldehyde 1,1’-CARBONYLDIIMIDAZOLE R-2-BENZYLOXY PROPIONIC ACID 1,1’-CARBONYLDI (1,2-4 TRIAZOLE) 4-HYDROXY BENZYL ALCOHOL 3-NITRO-2-METHYL ANILINE (2-Hydroxyphenyl)acetonitrile 4-Bromopyrazole 5-BROMO-2CYANO PYRIDINE 5,6-Dimethoxyindanone 5-broMo-2-chloro-N-cyclopentylpyriMidin-4-aMine 2-(Cyanocyclohexyl)acetic acid 4-methoxy-3,5-dinitropyridine 2-aminopropyl benzoate hydrochloride 1-(4-(aminomethyl)benzyl)urea hydrochloride tert-butyl 4- (ureidomethyl)benzylcarbamate diethyl 2-(2-((tertbutoxycarbonyl)amino) ethyl)malonate Ethyl-2-chloro((4-methoxyphenyl)hydrazono)acetateRelated products of tetrahydrofuran



You may like
-
 N-Vinylformamide 99%View Details
N-Vinylformamide 99%View Details
13162-05-5 -
 Chloro Uracil 1820-81-1 99%View Details
Chloro Uracil 1820-81-1 99%View Details
1820-81-1 -
 2-ethyl-6-methyl-3-hydroxypyridine succinate 99%View Details
2-ethyl-6-methyl-3-hydroxypyridine succinate 99%View Details
127464-43-1 -
 2-ETHYLPYRIDINE 100-71-0 99%View Details
2-ETHYLPYRIDINE 100-71-0 99%View Details
100-71-0 -
 7439-89-6 Electrolytic Iron Flakes 99.9% MaxView Details
7439-89-6 Electrolytic Iron Flakes 99.9% MaxView Details
7439-89-6 -
 7439-89-6 98.0% MinView Details
7439-89-6 98.0% MinView Details
7439-89-6 -
 Reduced Iron Powder 99.8% MaxView Details
Reduced Iron Powder 99.8% MaxView Details
7439-89-6 -
 Electrolytic Iron Powder 7439-89-6 99.8% MaxView Details
Electrolytic Iron Powder 7439-89-6 99.8% MaxView Details
7439-89-6