Olanzapine
Synonym(s):2-Methyl-4-(4-methyl-1-piperazinyl)- 10H-thieno[2,3-b][1,5]benzodiazepine;LY-170053;Olanzapine
- CAS NO.:132539-06-1
- Empirical Formula: C17H20N4S
- Molecular Weight: 312.43
- MDL number: MFCD00866702
- EINECS: 603-618-4
- SAFETY DATA SHEET (SDS)
- Update Date: 2025-12-28 08:51:53
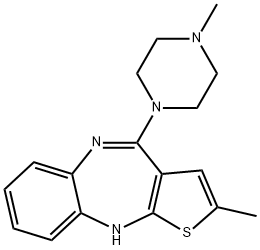
What is Olanzapine?
Absorption
Olanzapine presents a linear pharmacokinetic profile and, after daily administration, it reaches steady-state in about a week. Under the administration of a normal dosage of olanzapine, the steady-state plasma concentration does not seem to exceed 150 ng/ml with an AUC of 333 ng/h/ml.
The absorption of olanzapine is not affected by the concomitant administration of food. The pharmacokinetic profile of olanzapine is characterized by reaching peak plasma concentration of 156.9 ng/ml approximately 6 hours after oral administration.
Toxicity
The toxicity symptoms of olanzapine are known to include somnolence, mydriasis, blurred vision, respiratory depression, hypotension, extrapyramidal symptoms and anticholinergic effects. The overdosage effects in children are generally associated with more significant side effects.
Description
Olanzapine is a drug (Zyprexa) used to treat schizophrenia. In January 2007, the US Federal Circuit Court ruled that Eli Lilly''s olanzapine patent was valid and had been infringed by other drug makers.
Description
Zyprexa was launched in Canada, Germany, the UK and US as an antipsychotic agent. Prepared in three steps via the intermediate diazepinone, it is an atypical antipsychotic with a high affinity for dopaminergic and serotonergic receptors. Specifically, olazapine is a potent 5-HT2/D2 antagonist with anticholinergic activity. It has a greater antagonistic effect at 5-HT2a than at dopamine D2 receptors and in vivo is clozapine-like. Thus it is less likely to produce extrapyramidal side effects and does not produce any granulocytopenia. Its 10 metabolic products are all inactive.
Description
O lanzapine is classed as an atypical antipsychotic and is considered a firstline agent in the management of newly diagnosed psychosis. It has a similar mechanism of action to classical antipsychotics but with a lower incidence of adverse events. O lanzapine rarely produces hypotension and has less potential for QT prolongation. It is used in the management of delirium in ICU.
Chemical properties
Yellow Crystalline Powder
Originator
Lilly (USA)
The Uses of Olanzapine
A serotonin (5-HT2) and dopamine (D1/D2) receptor antagonist with anticholinergic activity. Antipsychotic.
The Uses of Olanzapine
intermediate for Imidacloprid, Indobufen, Nitroguanidine, Nalorphine, Tazarotene, Trovafloxacin
The Uses of Olanzapine
anti-ulcer, gastrointestinal-emptying agent enhances motility in the upper gastrointestinal tract
Indications
Olanzapine was initially used orally and intramuscularly for the chronic treatment of schizophrenia in patients over 13 years old and other psychiatric disorders such as bipolar I disorder including mixed or manic episodes.
Olanzapine is also indicated, in combination with lithium or valproate for the short-term treatment of acute manic or mixed episodes associated with bipolar I disorder in adults.
Background
Olanzapine is a thienobenzodiazepine classified as an atypical or second-generation antipsychotic agent. The second-generation antipsychotics were introduced in the 90s and quickly gained traction due to their impressive efficacy, reduced risk for extrapyramidal side effects and reduced susceptibility to drug-drug interactions. Olanzapine very closely resembles clozapine and only differs by two additional methyl groups and the absence of a chloride moiety. It was discovered by scientists at Eli Lilly and approved to be marketed in the US in 1996.
Definition
ChEBI: A benzodiazepine that is 10H-thieno[2,3-b][1,5]benzodiazepine substituted by a methyl group at position 2 and a 4-methylpiperazin-1-yl group at position 4.
Manufacturing Process
1. 2-Amino-5-methylthiophene-3-carbonitrile
A mixture of sulphur (217.8 g, 6.79 mol), propionaldehyde (472.5 g, 587 mL, 8.13 mol) and dimethylformamide (1350 m) was placed in a 5 liter flangenecked flask fitted with air stirrer, air condenser, thermometer and dropping funnel. Triethylamine (576 mL, 4.13 mol) was added dropwise over 30 minutes to the cooled stirred reaction mixture whilst maintaining the pot temperature between 5°-10°C with an ice-bath. After addition was complete the pot was allowed to warm up to 18°C over 50 minutes, keeping the mixture well stirred. Then a solution of malononitrile (450 g, 6.8 mol) in dimethylformamide (900 mL) was added dropwise over 70 minutes keeping the pot temperature around 20°C throughout the addition. After addition was complete the mixture was stirred at 15°-20°C for a further 45 minutes then sampled for TLC. The mixture was then poured onto ice (4 liters)/water (8 liters) with stirring and this caused the required product to precipitate. After 10 minutes the stirrer was switched off and the solid allowed to settle. The aqueous liquor was decanted away and the solid isolated by filtration. The isolated solid was well washed with water (de-ionised, 4 liters), then dried over night in vacuo at 70°-75°C to give the title compound (585 g), m.p. 100°C.
2. 2-(2-Nitroanilino)-5-methylthiophene-3-carbonitrile
To a stirred slurry of sodium hydride (14.4 g, 50% dispersion in oil, 0.3 mol) in dry tetrahydrofuran (50 mL) under nitrogen was added, dropwise, a solution of 2-fluoronitrobenzene (28.2 g, 0.2 mol) and 2-amino-5methylthiophene3-carbonitrile (27.6 g, 0.2 mol) in dry tetrahydrofuran (250 mL). The mixture was stirred at 25°C for 24 hours, poured onto cracked ice and extracted into dichloromethane (3 times 500 mL). The combined extracts were washed with 2 N hydrochloric acid (2 times 200 mL), water (2 times 200 mL), dried over magnesium sulphate and the solvent removed under reduced pressure. The residue was crystallised from ethanol to give the title compound, (35.2 g), m.p. 99°-102°C.
3. 4-Amino-2-methyl-10H-thieno[2,3-b][1,5]benzodiazepine, hydrochloride
To a stirred slurry of 2-(2-nitroanilino)-5-methylthiophene-3-carbonitrile (3 g, 0.011 mol) in ethanol (35 mL) at 50°C was added, over 10 minutes, a solution of anhydrous stannous chloride (6.95 g, 0.037 mol) in hydrochloric acid (26 mL, 5 M). The mixture was stirred under reflux for 1 hour, concentrated under reduced pressure and allowed to crystallise over night at 5°C. The salt was filtered, washed with a small amount of water, dried (4.3 g) m.p. >250°C, and used without further purification in the next stage.
4. 2-Methyl-10-(4-methyl-1-piperazinyl)-4H-thieno[2,3-b][1,5]benzodiazepine
Crude 4-amino-2-methyl-10H-thieno[2,3-b][1,5]benzodiazepine, hydrochloride (4.3 g) was refluxed in a mixture of N-methylpiperazine (15 mL), dimethylsulfoxide (20 mL) and toluene (20 mL) under a nitrogen atmosphere for 20 hours. The mixture was cooled to ca. 50°C, water (20 mL) added, and the product allowed to crystallise at 5°C over night. The product was filtered and crystallised from acetonitrile (30 mL) to give the title compound (1.65 g) m.p. 195°C. The structure of the compound was confirmed spectroscopically
brand name
Zyprexa (Lilly).
Therapeutic Function
Antipsychotic
General Description
Olanzapine, 2-methyl-4-(4-methyl-1-piperazinyl)-10H-thieno[2,3-b][1,5] benzodiazepine (Zyprexa), isa yellow crystalline solid that is essentially insoluble in water.Olanzapine orally disintegrating tablets (Zyprexa Zydis) is asolid dosage form that immediately disintegrates when exposedto saliva. This product is useful for elderly patients whohave difficulty in swallowing. An injectable form, olanzapinefor injection (Zyprexa IM), is indicated for agitation associatedwith schizophrenia or bipolar I mania. Olanzapine iscombined with fluoxetine (Symbyax) for use in depressionthat is associated with bipolar I disorder. Peak concentrationsof oral olanzapine are reached at 6 hours after oral administration,and absorption of the compound is not affected byfood.
Olanzapine binds with high affinity at DA D2, 5-HT2A,5-HT2C, 5-HT6,α1, and H1 histamine receptors. The effectsof olanzapine for the treatment of schizophrenia are presumablymediated through antagonism at D2 and 5-HT2Areceptors.The use of olanzapine in acute maniathat is associated with bipolar I disorder is thought to bemediated by antagonism at D2 and other monamine receptors.Additionally, olanzapine is postulated to produce itsmood stabilizing and antidepressant effects through 5-HT2Areceptor blockade and increased cortical DA and NE concentrations.Several studies have investigated the effectof olanzapine-induced weight gain and new-onset type 2diabetes.In a comparison study with risperidone,olanzapine was shown to have a greater risk of producingdyslipidemia and type 2 diabetes.
Biochem/physiol Actions
Olanzapine is a 5-HT2 serotonin and D1/D2 dopamine receptor antagonist.
Pharmacokinetics
The effect of olanzapine in the D2 receptor is reported to produce the positive effects of this drug such as a decrease in hallucinations, delusions, disorganized speech, disorganized thought, and disorganized behavior. On the other hand, its effect on the serotonin 5HT2A receptor prevents the onset of anhedonia, flat affect, alogia, avolition and poor attention. Based on the specific mechanism of action, olanzapine presents a higher affinity for the dopamine D2 receptor when compared to the rest of the dopamine receptor isotypes. This characteristic significantly reduces the presence of side effects.
Clinical Use
The thienobenzodiazepine olanzapine is an effective atypical antipsychotic agent that is close in structure to clozapine but has a somewhat different neuropharmacological profile, in that it is a more potent antagonist at dopamine D2 and, especially, serotonin 5-HT2A receptors. Olanzapine is well absorbed after oral administration and is metabolized mainly by CYP1A2 to inactive metabolites, with a variable half-life of approximately 20 to 50 hours.
in vitro
binding studies showed that olanzapine interacted with keyreceptorsof interest in schizophrenia, exihibiting a nanomolar affinity for dopaminergic, serotonergic, alpha 1-adrenergic, and muscarinic receptors [1].
in vivo
olanzapine was a potent antagonist at dareceptorsand 5-ht receptors, but showed weaker activity at alpha-adrenergic and muscarinic receptors [1].administration of olanzapine at 0.5, 3 and 10 mg/kg (s.c.) increased the extracellulardopamine(da) and norepinephrine (ne) levels in all three brain areas in a dose-dependent manner.the increases reached peaks 60-90 min after olanzapine administration and lasted for at least 2 h. the highest da increases in the acb and cpu were induced by olanzapine at 3 mg/kg but at 10 mg/kg in the pfc while the highest ne increase in the pfc (414% ± 40) induced by 10 mg/kg olanzapine [2].in macaque monkeys, olanzapine treatment resulted in an 8-11% reduction in mean fresh brain weights as well as left cerebrum fresh weights and volumes [3].
Drug interactions
Potentially hazardous interactions with other drugs
Anaesthetics: enhanced hypotensive effect.
Analgesics: increased risk of convulsions with
tramadol; enhanced hypotensive and sedative
effects with opioids; increased risk of ventricular
arrhythmias with methadone.
Anti-arrhythmics: increased risk of ventricular
arrhythmias.
Antibacterials: concentration possibly increased by
ciprofloxacin.
Antidepressants: fluvoxamine increases concentration
of olanzapine; increased concentration of tricyclics.
Antiepileptics: antagonism (convulsive threshold
lowered); carbamazepine increases metabolism
of olanzapine; increased risk of neutropenia with
valproate.
Antimalarials: avoid with artemether/lumefantrine.
Antipsychotics: increased risk of ventricular
arrhythmias with risperidone.
Antivirals: concentration reduced by ritonavir -
consider increasing olanzapine dose.
Anxiolytics and hypnotics: increased sedative
effects; increased risk of hypotension, bradycardia
and respiratory depression with IM olanzapine and
parenteral benzodiazepines.
Atomoxetine: increased risk of ventricular
arrhythmias.
Cytotoxics: increased risk of ventricular arrhythmias
with arsenic trioxide.
Metabolism
Olanzapine is greatly metabolized in the liver, which represents around 40% of the administered dose, mainly by the activity of glucuronide enzymes and by the cytochrome P450 system. From the CYP system, the main metabolic enzymes are CYP1A2 and CYP2D6. As part of the phase I metabolism, the major circulating metabolites of olanzapine, accounting for approximate 50-60% of this phase, are the 10-N-glucuronide and the 4'-N-desmethyl olanzapine which are clinically inactive and formed by the activity of CYP1A2. On the other hand, CYP2D6 catalyzes the formation of 2-OH olanzapine and the flavin-containing monooxygenase (FMO3) is responsible for N-oxide olanzapine.
On the phase II metabolism of olanzapine, UGT1A4 is the key player by generating direct conjugation forms of olanzapine.
Metabolism
Olanzapine is extensively metabolised in the liver, mainly
by direct glucuronidation and by oxidation mediated
through the cytochrome P450 isoenzymes CYP1A2, and,
to a lesser extent, CYP2D6. The 2 major metabolites,
10-N-glucuronide and 4′-N-desmethyl olanzapine,
appear to be inactive.
About 57% of a dose is excreted in the urine, mainly as
metabolites, and about 30% appears in the faeces.
Storage
+4°C
References
[1]. bymaster fp1,rasmussen k,calligaro do,nelson dl,delapp nw,wong dt,moore na. in vitro and in vivo biochemistry of olanzapine: a novel, atypical antipsychotic drug.j clin psychiatry.1997;58suppl 10:28-36.
[2]. li xm1,perry kw,wong dt,bymaster fp. olanzapine increases in vivodopamineand norepinephrine release in rat prefrontal cortex, nucleus accumbens and striatum.psychopharmacology (berl).1998 mar;136(2):153-61.
[3]. dorph-petersen ka1,pierri jn,perel jm,sun z,sampson ar,lewis da. the influence of chronic exposure to antipsychotic medications on brain size before and after tissue fixation: a comparison of haloperidol and olanzapine in macaque monkeys.neuropsychopharmacology.2005 sep;30(9):1649-61.
Properties of Olanzapine
| Melting point: | 195°C |
| Boiling point: | 476.0±55.0 °C(Predicted) |
| Density | 1.32±0.1 g/cm3(Predicted) |
| Flash point: | 2℃ |
| storage temp. | 2-8°C |
| solubility | DMSO: >15mg/mL |
| form | powder |
| pka | 7.78±0.20(Predicted) |
| color | yellow |
| Merck | 14,6822 |
| BRN | 7655141 |
| CAS DataBase Reference | 132539-06-1(CAS DataBase Reference) |
| NIST Chemistry Reference | Olanzapine(132539-06-1) |
Safety information for Olanzapine
| Signal word | Warning |
| Pictogram(s) |
 Exclamation Mark Irritant GHS07 |
| GHS Hazard Statements |
H315:Skin corrosion/irritation H319:Serious eye damage/eye irritation |
| Precautionary Statement Codes |
P264:Wash hands thoroughly after handling. P264:Wash skin thouroughly after handling. P280:Wear protective gloves/protective clothing/eye protection/face protection. P302+P352:IF ON SKIN: wash with plenty of soap and water. P305+P351+P338:IF IN EYES: Rinse cautiously with water for several minutes. Remove contact lenses, if present and easy to do. Continuerinsing. P332+P313:IF SKIN irritation occurs: Get medical advice/attention. P337+P313:IF eye irritation persists: Get medical advice/attention. |
Computed Descriptors for Olanzapine
Olanzapine manufacturer
Remedy Labs
New Products
4,4-Difluoropiperidine hydrochloride tert-butyl 9-methoxy-3-azaspiro[5.5]undecane-3-carboxylate Indole Methyl Resin N-Isopropylurea N,N-Dicyclohexylcarbodiimide(DCC) MELDRUMS ACID 5-METHYLISOXAZOLE-4-CARBOXYLIC ACID Magnessium Bis glycinate Zinc ascorbate 1-bromo-2-butyne 2-acetamidophenol 9(10H)-anthracenone Erythrosin B, 4-Piperidinopiperidine 2-((4-morpholinophenylamino) (methylthio) methylene) malononitrile 2,4-dihydroxybenzaldehyde 3-(4-morpholinophenylamino)-5-amino-1H-pyrazole-4-carbonitrile Methyl 2-methylquinoline-6-carboxylate 2,6-dichloro-4-nitropyridine 4-Bromo-2-chlorobenzonitrile 2-(benzylamino)acetic acid hydrochloride 4-(tert-Butoxycarbonylamino)but- 2-ynoic acid 3,4-dihydro-2H-benzo[b][1,4]dioxepine 1-Phenyl-1-cycloprppanecarboxylicacidRelated products of tetrahydrofuran




You may like
-
 Olanzapine 132539-06-1 98%View Details
Olanzapine 132539-06-1 98%View Details
132539-06-1 -
 Olanzapine 98%View Details
Olanzapine 98%View Details -
 Olanzapine 132539-06-1 98%View Details
Olanzapine 132539-06-1 98%View Details
132539-06-1 -
 Olanzapine 98% (HPLC) CAS 132539-06-1View Details
Olanzapine 98% (HPLC) CAS 132539-06-1View Details
132539-06-1 -
 Olanzapine 98% CAS 132539-06-1View Details
Olanzapine 98% CAS 132539-06-1View Details
132539-06-1 -
 Olanzapine 99% (HPLC) CAS 132539-06-1View Details
Olanzapine 99% (HPLC) CAS 132539-06-1View Details
132539-06-1 -
 Olanzapine Api PowderView Details
Olanzapine Api PowderView Details
132539-06-1 -
 Olanzapine Api Powder IP/BP/USP/EPView Details
Olanzapine Api Powder IP/BP/USP/EPView Details
132539-06-1
