Azaperone
Synonym(s):1-(4-Fluorophenyl)-4-(4-(2-pyridinyl)-1-piperazinyl)-1-butanone;4′-Fluoro-4-[4-(2-pyridyl)-1-piperazinyl]butyrophenone
- CAS NO.:1649-18-9
- Empirical Formula: C19H22FN3O
- Molecular Weight: 327.4
- MDL number: MFCD00866692
- EINECS: 216-715-8
- SAFETY DATA SHEET (SDS)
- Update Date: 2024-11-19 23:02:33
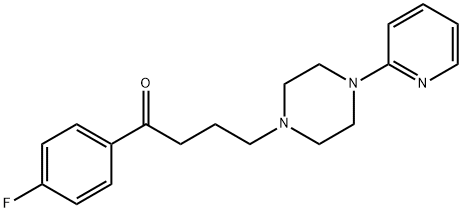
What is Azaperone?
Description
Azaperone is a sedative used in veterinary medicine to avoid mortality of pigs during transportation. This alternative substance to chlorpromazine is a sensitizer and a photosensitizer.
Chemical properties
Off-White Solid
Physical properties
A butyrophenone neuroleptic, azaperone occurs as a white to yellowish-white macrocrystalline powder with a melting point between 90-95°C. It is practically insoluble in water; 1 gram is soluble in 29 mL of alcohol.
Azaperone may also be known as azaperonum, R-1929, Stresnil®, or Suicalm®.
Originator
Azaperone,Dayang Chemicals Co. Ltd.
The Uses of Azaperone
Sedative; tranquilizer.
The Uses of Azaperone
H1 antihistamine (nonsedating); leucotriene synthesis blocker
What are the applications of Application
Azaperone is an α-adrenergic, dopamine and histamine receptor inhibitor
Definition
ChEBI: An N-arylpiperazine that is 2-(piperazin-1-yl)pyridine in which the amino hydrogen is replaced by a 3-(4-fluobenzoyl)propyl group. Used mainly as a tranquiliser for pigs and elephants.
Indications
Azaperone is officially indicated for the "control of aggressiveness when mixing or regrouping weanling or feeder pigs weighing up to 36.4 kg" (Package Insert, Stresnil®—P/M; Mallinckrodt). It is also used clinically as a general tranquilizer for swine, to allow piglets to be accepted by aggressive sows, and as a preoperative agent prior to general anesthesia or cesarean section with local anesthesia.
Azaperone has been used as a neuroleptic in horses, but some horses develop adverse reactions (sweating, muscle tremors, panic reaction, CNS excitement) and IV administration has resulted in significant arterial hypotension. Because of these effects, most clinicians avoid the use of this drug in equines.
Manufacturing Process
A mixture of γ-chloro-4'-fluorobutyrophenone and 1-(2'-pyridyl)piperazine is heated on an oil bath. The mixture is then boiled in diisopropyl ether and the precipitates is collected and boiled with water and benzene. The benzene layer is treated with activated charcoal, added to the ethereal filtrate, and evaporated to give a residue which is taken up in diisopropyl ether. After cooling an oil is precipitated, which after decantation and drying yields 1-[γ- (4'-fluorobenzoyl)propyl]-4-(2'-pyridyl)piperazine.
brand name
Stresnil (Janssen Pharmaceutica, Belgium); Suicalm (Janssen Pharmaceutica, Belgium).
Therapeutic Function
Neuroleptic, Hypnotic
Contact allergens
Azaperone is a sedative used in veterinary medicine to avoid mortality of pigs during transportation. This alternative substance to chlorpromazine is a sensitizer and a photosensitizer.
Pharmacology
The butyrophenones as a class cause tranquilization and sedation (sedation may be less than with the phenothiazines), anti-emetic activity, reduced motor activity, and inhibition of CNS catecholamines (dopamine, norepinephrine). Azaperone appears to have minimal effects on respiration and may inhibit some of the respiratory depressant actions of general anesthetics. A slight reduction of arterial blood pressure has been measured in pigs after IM injections of azaperone, apparently due to slight alpha-adrenergic blockade. Azaperone has been demonstrated to prevent the development of halothane-induced malignant hyperthermia in susceptible pigs. Preliminary studies have suggested that the effects of butyrophenones may be antagonized by 4-aminopyridine.
Pharmacokinetics
Minimal information was located regarding actual pharmacokinetic parameters, but the drug is considered to have a fairly rapid onset of action following IM injections in pigs (5-10 minutes) with a peak effect at approximately 30 minutes post injection. It has a duration of action of 2-3 hours in young pigs and 3-4 hours in older swine. The drug is metabolized in the liver with 13% of it excreted in the feces. At 16 hours post-dose, practically all of the drug is eliminated from the body; however in the UK a 10-day slaughter withdrawal has been assigned.
Side Effects
Transient salivation, piling, panting and shivering have been reported in pigs. Pigs should be left undisturbed after injection (for approximately 20 minutes) until the drug's full effects have been expressed; disturbances during this period may trigger excitement.
Azaperone has minimal analgesic effects and is not a substitute for appropriate anesthesia or analgesia. Doses above 1 mg/kg may cause the penis to be extruded in boars.
Safety Profile
Poison by ingestion, intravenous, intraperitoneal, and subcutaneous routes. When heated to decomposition it emits very toxic fumes of Fand NOx.
Veterinary Drugs and Treatments
Azaperone is officially indicated for the “control of aggressiveness
when mixing or regrouping weanling or feeder pigs weighing up to
36.4 kg” (Package Insert, Stresnil?—P/M; Mallinckrodt). It is also
used clinically as a general tranquilizer for swine, to allow
piglets to
be accepted by aggressive sows, and as a preoperative agent prior to
general anesthesia or cesarean section with local anesthesia.
Azaperone has been used as a neuroleptic in horses, but some
horses develop adverse reactions (sweating, muscle tremors, panic
reaction, CNS excitement) and IV administration has resulted in
significant arterial hypotension. Because of these effects, most clinicians
avoid the use of this drug in equines.
Overdosage
Overdoses (>1 mg/kg) in boars may cause penis extrusion leading to damage.
storage
Azaperone should be stored at controlled room temperature (15-25°C) and away from light. Do not store above 25°C. Once the vial is opened it should be used within 28 days. No information was located regarding mixing azaperone with other compounds.
Precautions
When used as directed, the manufacturer reports no contraindications (other than for slaughter withdrawal) for the drug. It should not be given IV as a significant excitatory phase may be seen in pigs. Avoid use in very cold conditions as cardiovascular collapse may occur secondary to peripheral vasodilation.
Do not exceed dosing recommendation in boars as the drug may cause the penis to be extruded.
Because Vietnamese Pot Bellied pigs may have delayed absorption due to sequestration of the drug in body fat, re-dose with extreme caution; deaths have resulted after repeat dosing.
Properties of Azaperone
| Melting point: | 87-89?C |
| Boiling point: | 236°C (rough estimate) |
| Density | 1.1513 (estimate) |
| storage temp. | Sealed in dry,2-8°C |
| solubility | DMF: 15 mg/ml; DMSO: 15 mg/ml; Ethanol: 10 mg/ml; PBS (pH 7.2): insol |
| pka | 8.43±0.19(Predicted) |
| form | Solid |
| form | neat |
| color | Light yellow to yellow |
| CAS DataBase Reference | 1649-18-9(CAS DataBase Reference) |
| NIST Chemistry Reference | Azaperone(1649-18-9) |
Safety information for Azaperone
| Signal word | Danger |
| Pictogram(s) |
 Skull and Crossbones Acute Toxicity GHS06 |
| GHS Hazard Statements |
H301:Acute toxicity,oral |
Computed Descriptors for Azaperone
| InChIKey | XTKDAFGWCDAMPY-UHFFFAOYSA-N |
New Products
4-Fluorophenylacetic acid 4-Methylphenylacetic acid N-Boc-D-alaninol N-BOC-D/L-ALANINOL Tert-butyl bis(2-chloroethyl)carbamate 3-Morpholino-1-(4-nitrophenyl)-5,6-dihydropyridin- 2(1H)-one Furan-2,5-Dicarboxylic Acid Tropic acid S-2-CHLORO PROPIONIC ACID ETHYL ISOCYANOACETATE 2-Bromo-1,3-Bis(Dimethylamino)Trimethinium Hexafluorophosphate (6-METHYL-[1,3]DITHIOLO[4,5-b]QUINOXALIN-2-ONE INDAZOLE-3-CARBOXYLIC ACID 4-IODO BENZOIC ACID (2-Hydroxyphenyl)acetonitrile 4-Bromopyrazole 5,6-Dimethoxyindanone 2-(Cyanocyclohexyl)acetic acid 4-methoxy-3,5-dinitropyridine 2-aminopropyl benzoate hydrochloride 1-(4-(aminomethyl)benzyl)urea hydrochloride diethyl 2-(2-((tertbutoxycarbonyl)amino) ethyl)malonate tert-butyl 4- (ureidomethyl)benzylcarbamate Ethyl-2-chloro((4-methoxyphenyl)hydrazono)acetateRelated products of tetrahydrofuran


You may like
-
 Azaperone 99% (HPLC) CAS 1649-18-9View Details
Azaperone 99% (HPLC) CAS 1649-18-9View Details
1649-18-9 -
 Azaperone CAS 1649-18-9View Details
Azaperone CAS 1649-18-9View Details
1649-18-9 -
 1975-50-4 98%View Details
1975-50-4 98%View Details
1975-50-4 -
 2-HYDROXY BENZYL ALCOHOL 98%View Details
2-HYDROXY BENZYL ALCOHOL 98%View Details
90-01-7 -
 2-Chloro-1,3-Bis(Dimethylamino)Trimethinium Hexafluorophosphate 221615-75-4 98%View Details
2-Chloro-1,3-Bis(Dimethylamino)Trimethinium Hexafluorophosphate 221615-75-4 98%View Details
221615-75-4 -
 61397-56-6 CIS BROMO BENZOATE 98%View Details
61397-56-6 CIS BROMO BENZOATE 98%View Details
61397-56-6 -
 14714-50-2 (2-Hydroxyphenyl)acetonitrile 98+View Details
14714-50-2 (2-Hydroxyphenyl)acetonitrile 98+View Details
14714-50-2 -
 118753-70-1 98+View Details
118753-70-1 98+View Details
118753-70-1