Avridine
Synonym(s):CP 20961;N,N-Dioctadecyl-N′,N′-bis(2-hydroxyethyl)-1,3-diaminopropane;N,N-Dioctadecyl-N′,N′-bis(2-hydroxyethyl)propanediamine
- CAS NO.:35607-20-6
- Empirical Formula: C43H90N2O2
- Molecular Weight: 667.19
- MDL number: MFCD00673242
- SAFETY DATA SHEET (SDS)
- Update Date: 2025-08-21 22:16:43
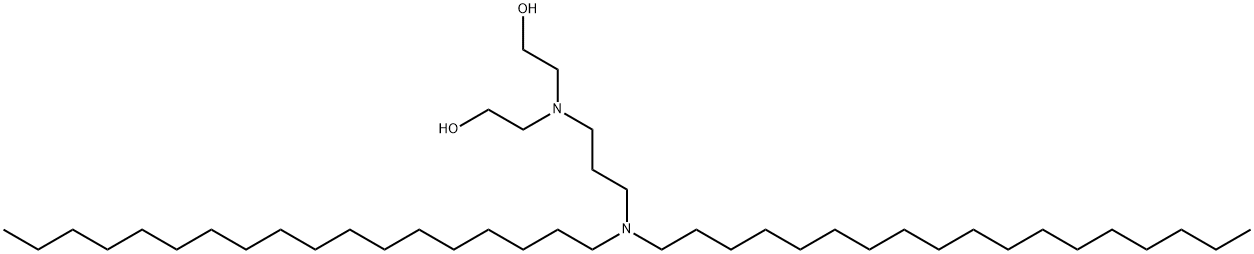
What is Avridine?
Chemical properties
Off-White to Pale Yellow Solid
Originator
Avridine,Chemical
The Uses of Avridine
A potent synthetic non-immunogenic adjuvant that can induce arthritis in most rat strains; immunomodulator and interferon-inducing.
What are the applications of Application
CP 20961 is a non-immunogenic adjuvant
Definition
ChEBI: Avridine is an amino alcohol.
Manufacturing Process
N,N-Dioctadecyl-1,3-propanediamine:
A). A two-gallon autoclave is charged with 3-(dioctadecylamino)propionitrile
(100 g), ethanol (3750 ml) containing anhydrous ammonia (100 g) and Raney
nickel (20 g dry basis) and purged with nitrogen, then with hydrogen. It is
then sealed and the hydrogen pressure raised to 250 psi. The autoclave is
agitated, the temperature raised to 70°C and the mixture held at this
temperature for 1.5 hours at which time hydrogen absorption has ceased. The
autoclave is cooled to 20°C, vented, and the contents removed. The catalyst
is filtered off, washed with ethanol, and the combined washings and reaction
mixture concentrated in vacuum to a viscous green-yellow oil (82 g) which
solidified upon standing, MP: 39°-41°C.
3-(Dioctadecylamino)propionitrile is prepared by refluxing a mixture of
dioctadecylamine (200 g) and acrylonitrile (1903.8 ml) for eighteen hours.
The mixture is then concentrated to a waxy semi-solid which is slurried in
acetone, filtered, and air dried overnight.
B). The monoacyl derivatives of N,N-dioctadecyl-1,3-propanediamine are
prepared as follows:
To a solution of methylene chloride (500 ml per 0.1 mole of reactants)
containing equimolar amounts of N,N-dioctadecyl-1,3-propanediamine and
triethylamine and cooled in an ice-bath is added an equimolar amount of the
appropriate acyl chloride in methylene chloride (25 ml per 0.1 mole of acyl
chloride) over a period of 15 minutes. The mixture is stirred for ten minutes
then brought to room temperature and stirred for one hour. The methylene
chloride phase is separated and extracted with water (3x25 ml). The water is in turn extracted with methylene chloride (2x25 ml) and the combined
methylene chloride phases dried (Na2SO4) then evaporated under reduced
pressure. The residue is taken up in benzene and the solution passed through
a silica gel column. The column is eluted with benzene, then with benzene
containing increasing amounts of ethyl acetate, e.g., 5, 10, 25, and 50 %.
The eluate is subjected to thin layer chromatography (ethyl acetate) and
those fractions, which show only one spot, combined and evaporated to give
N,N-dioctadecyl-N',N'-bis(2-hydroxyethyl)propanediamine M.P. 48.5°-49°C.
Therapeutic Function
Antiviral
Biological Activity
Non-immunogenic adjuvant; used to induce arthritis in Lewis (LEW) and DA rats.
Properties of Avridine
| Melting point: | 39-40°C |
| Boiling point: | 717.6±40.0 °C(Predicted) |
| Density | 0.892±0.06 g/cm3(Predicted) |
| storage temp. | Store at RT |
| solubility | DMSO: ≥5mg/mL (with warming to 60 °C for 5 minutes) |
| pka | 14.41±0.10(Predicted) |
| form | powder |
| color | white to off-white |
Safety information for Avridine
Computed Descriptors for Avridine
New Products
4,4-Difluoropiperidine hydrochloride tert-butyl 9-methoxy-3-azaspiro[5.5]undecane-3-carboxylate Indole Methyl Resin N-Isopropylurea N,N-Dicyclohexylcarbodiimide(DCC) MELDRUMS ACID 5-METHYLISOXAZOLE-4-CARBOXYLIC ACID Magnessium Bis glycinate Zinc ascorbate 1-bromo-2-butyne 2-acetamidophenol 9(10H)-anthracenone Erythrosin B, 4-Piperidinopiperidine 2-((4-morpholinophenylamino) (methylthio) methylene) malononitrile 2,4-dihydroxybenzaldehyde 3-(4-morpholinophenylamino)-5-amino-1H-pyrazole-4-carbonitrile Methyl 2-methylquinoline-6-carboxylate 2,6-dichloro-4-nitropyridine 4-Bromo-2-chlorobenzonitrile 2-(benzylamino)acetic acid hydrochloride 4-(tert-Butoxycarbonylamino)but- 2-ynoic acid 3,4-dihydro-2H-benzo[b][1,4]dioxepine 1-Phenyl-1-cycloprppanecarboxylicacidRelated products of tetrahydrofuran



You may like
-
 Avridine CAS 35607-20-6View Details
Avridine CAS 35607-20-6View Details
35607-20-6 -
 3-(4-amino-1-oxoisoindolin-2-yl)-1-methylpiperidine-2,6-dione 98%View Details
3-(4-amino-1-oxoisoindolin-2-yl)-1-methylpiperidine-2,6-dione 98%View Details -
 614-19-7 98%View Details
614-19-7 98%View Details
614-19-7 -
 3112-85-4 Methyl phenyl sulfone 98%View Details
3112-85-4 Methyl phenyl sulfone 98%View Details
3112-85-4 -
 20677-73-0 (2,2-diethoxyethyl)methylamine 98%View Details
20677-73-0 (2,2-diethoxyethyl)methylamine 98%View Details
20677-73-0 -
 3-(4-(hydroxyamino)-1-oxoisoindolin-2-yl)piperidine-2,6-dione 98%View Details
3-(4-(hydroxyamino)-1-oxoisoindolin-2-yl)piperidine-2,6-dione 98%View Details -
 57381-49-4 2-bromo-4-chlorobenzonitrile 98%View Details
57381-49-4 2-bromo-4-chlorobenzonitrile 98%View Details
57381-49-4 -
 4,6-dichloropyrimidine-5-carbaldehyde 98%View Details
4,6-dichloropyrimidine-5-carbaldehyde 98%View Details
5305-40-8
