Atovaquone
Synonym(s):Atovaquone;Mepron;trans-2-[4-(4-Chlorophenyl)cyclohexyl]-3-hydroxy-1,4-naphthalenedione
- CAS NO.:95233-18-4
- Empirical Formula: C22H19ClO3
- Molecular Weight: 366.84
- MDL number: MFCD00889188
- EINECS: 000-000-0
- SAFETY DATA SHEET (SDS)
- Update Date: 2025-12-26 16:58:18
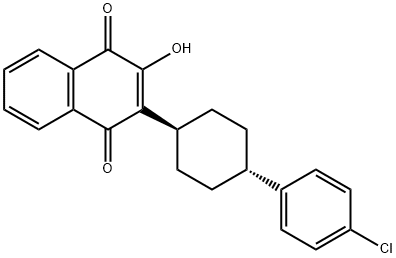
What is Atovaquone?
Absorption
The bioavailability of atovaquone is low and variable and is highly dependent on formulation and diet. Bioavailability of the suspension increases two-fold when administered with meals. When administered with food, bioavailability is approximately 47%. Without food, the bioavailability is 23%.
Toxicity
The median lethal dose is higher than the maximum oral dose tested in mice and rats (1825 mg/kg per day). Overdoses up to 31,500 mg of atovaquone have been reported. In one such patient who also took an unspecified dose of dapsone, methemoglobinemia occurred. Rash has also been reported after overdose.
Description
Atovaquone is an orally active antiprotozoal agent indicated for patients with mild to moderate AIDS-associated Pneumocystis carinii pneumonia who are intolerant to the fist-line therapy of trimethoprim-sulfamethoxazole. It is also under investigation as a treatment for malaria and AIDS-associated toxoplasmosis.
Chemical properties
Yellow to Orange Crystalline Solid
Originator
Wellcome (United Kingdom)
The Uses of Atovaquone
Atovaquone inhibits the cytochrome bc(1) complex via interactions with the Rieske iron-sulfur protein and cytochrome b in the ubiquinol oxidation pocket. In addition to its use as a treatment for toxoplasmosis, atovaquone has antimalarial properties and prevents pneumocystis pneumonia post-renal transplant.
The Uses of Atovaquone
Hydroxynaphthoquinone derivative that inhibits mitochondrial electron transport. Antipneumocystic
The Uses of Atovaquone
beta-adrenergic blocker
Indications
For the treatment or prevention of Pneumocystis carinii pneumonia in patients who are intolerant to trimethoprim-sulfamethoxazole (TMP-SMX). Also indicated for the acute oral treatment of mild to moderate PCP in patients who are intolerant to TMP-SMX.
Background
Atovaquone is a hydroxynaphthoquinone, or an analog of ubiquinone, that has antimicrobial and antipneumocystis activity. It is being used in antimalarial protocols.
What are the applications of Application
Atovaquone-d4 is a labeled Hydroxynaphthoquinone derivative that inhibits mitochondrial electron transport
What are the applications of Application
Atovaquone is an inhibitor of mitochondrial electron transport
Definition
ChEBI: A naphthoquinone compound having a 4-(4-chlorophenyl)cyclohexyl group at the 2-position and a hydroxy substituent at the 3-position.
Indications
Atovaquone is a naphthoquinone whose mechanism of
action involves inhibition of the mitochondrial electron
transport system in the protozoa. Malaria parasites depend
on de novo pyrimidine biosynthesis through dihydroorotate
dehydrogenase coupled to electron transport.
Plasmodia are unable to salvage and recycle
pyrimidines as do mammalian cells.
Atovaquone is poorly absorbed from the gastrointestinal
tract, but absorption is increased with a fatty
meal. Excretion of the drug, mostly unchanged, occurs
in the feces.The elimination half-life is 2 to 3 days. Low
plasma levels persist for several weeks. Concurrent administration
of metoclopramide, tetracycline, or rifampin
reduces atovaquone plasma levels by 40 to 50%.
Atovaquone has good initial activity against the
blood but not the hepatic stage of P. vivax and P. ovale
malaria parasites. It is effective against erythrocytic and
exoerythrocytic P. falciparum, and therefore, daily suppressive
doses need to be taken for only 1 week upon
leaving endemic areas.When used alone, it has an unacceptable
(30%) rate of recrudescence and selects for resistant
organisms. It and proguanil are synergistic when
combined and no atovaquone resistance is seen. This
combination (Malarone) is significantly more effective
than mefloquine, amodiaquine, chloroquine, and combinations
of chloroquine, pyrimethamine, and sulfadoxine.
In addition to using the combination of atovaquone
and proguanil for the treatment and prophylaxis of P.
falciparum malaria, atovaquone is also used for the
treatment and prevention of P. carinii pneumonia and
babesiosis therapy.
Atovaquone is well tolerated and produces only
rare instances of nausea, vomiting, diarrhea, abdominal
pain, headache, and rash of mild to moderate intensity.
Manufacturing Process
Preparation of intermediate 4-(4-chlorophenyl)cyclohexane-1-carboxylic acid
was needed at first. It was made as follows: acetyl chloride (30 g), aluminium
chloride (60 g) in carbon disulfide (120 ml) were stirred at -50°C. Cyclohexen
(30 g) previously cooled to -50°C was added dropwise during 10 minutes and
the mixture was stirred for 60 minutes at -50°C. The solvent was decanted
and 300 ml chlorobenzene was added, the so-obtained solution heated at
40°C for 3 hours with stirring, poured onto a mixture of ice and concentrated
hydrochloric acid and the organic layer washed with 2 M HCl, 2 M NaOH and
water, dried over anhydrous Na2SO4. The product was distilled in vacuo, the
fraction boiling at 140°-154°C (0.1 mm Hg) collected, diluted with an equal
volume of petroleum ether, cooled to -6°C and a stream of nitrogen gas
bubbled through.
3.1 g above obtained hexahydroacetophenone was dissolved in dioxan (15 ml)
and the fresh preparated hypobromite (8 ml) in a solution of NaOH (6.2 g) in
water (42 ml) at 0°C was added at below 20°C. The mixture was stirred at
ambient temperature for 6 hours then allowed to stand overnight. Excess
hypobromite was destroyed with sodium metabisulphite, cooled and then
acidified to give a colourless solid. It was filtered off, washed with water, dried
and recrystallysed from ethanol to give 4-(4-chlorophenyl)cyclohexane-1-
carboxylic acid, m.p. 254°-256°C. A mixture of this acid, 2-chloro-1,4-
naphthoquinone and silver nitrate was added to ammonium persulfate to give
the corresponding naphthoquinone which was saponificated with KOH and was
yielded 2-trans-4-(p-chlorophenyl)cyclohexyl)-3-hydroxy-1,4 naphthoquinone,
m.p. 216°-219°C, shown by NMR to be the pure trans isomer.
brand name
Mepron (GlaxoSmithKline).
Therapeutic Function
Antiprotozoal
Antimicrobial activity
It is active against erythrocytic, liver and sexual stages of malaria parasites. It shows synergy with proguanil and tetracyclines in vitro. It is also active against Babesia spp. and both tachyzoites and cysts of Tox. gondii. Pn. jirovecii is sensitive in vitro at 0.1–3.0 mg/L and high doses are effective in the rat.
Acquired resistance
Point mutations on parasite cytochrome b, in particular at codon 268, cause resistance and readily occur when the drug is used alone. The rapid selection of resistance led to the development of the synergistic combination with proguanil. Failure of Pn. jirovecii prophylaxis has also been associated with cytochrome b mutations.
General Description
Pharmaceutical secondary standards for application in quality control, provide pharma laboratories and manufacturers with a convenient and cost-effective alternative to the preparation of in-house working standards.
Atovaquone belongs to the class of naphthoquinones with a broad-spectrum activity against parasitic infections including malaria, toxoplasmosis and Pneumocystis pneumonia. It is a potent antiprotozoal compound which aids in collapsing the mitochondrial membrane potential in a malaria parasite.
Pharmaceutical Applications
A hydroxynaphthoquinone. Available as the trans isomer (which is more active than the cis form) for oral use. It is insoluble in water.
Biochem/physiol Actions
Atovaquone is an anti-protozoal mitochondrial electron transport inhibitor; Antimalarial; Antipneumocystic, and has also been used to treat toxoplasmosis. It is an analog of protozoan mitochondrial protein ubiquinone, and acts by inhibiting the cytochrome bc(1) complex via interactions with the Rieske iron-sulfur protein and cytochrome b in the ubiquinol oxidation pocket.
Mechanism of action
Atovaquone is thought to produce its antiparasitic action by virtue of its ability to inhibit the mitochondrial respiratory chain. More specifically, atovaquone is a ubiquinone reductase inhibitor, inhibiting at the cytochrome bc1 complex. This action leads to a collapse of the mitochondrial membrane potential. The compound shows stereospecific inhibition, with the trans isomer being more active than the cisisomer.
Pharmacokinetics
Atovaquone is a highly lipophilic drug that closely resembles the structure ubiquinone. Its inhibitory effect being comparable to ubiquinone, atovaquone can act by selectively affecting mitochondrial electron transport and parallel processes such as ATP and pyrimidine biosynthesis in atovaquone-responsive parasites. Cytochrome bc1 complex (complex III) seems to serve as a highly discriminating molecular target for atovaquone in Plasmodia. There is no significant risk for myelosuppression associated with atovaquone, making this drug a beneficial therapeutic agent for recipients of bone marrow transplantation.
Pharmacokinetics
Oral absorption: Poor
Cmax 750 mg oral: 27 mg/L (steady state)
Plasma half-life: 70 h
Plasma protein binding: >99%
It is highly lipophilic and is poorly absorbed from the gastrointestinal
tract following oral administration. Bioavailability is
improved when administered with meals, particularly those
with a high fat content. Steady-state plasma concentrations
are up to 50% lower in AIDS patients than in asymptomatic
HIV-positive cases and the elimination half-life is lower (55 h)
in patients with AIDS. The concentration in CSF is <1% of
the plasma level. Unlike some other naphthoquinones it is not
metabolized by human liver microsomes. Combinations with
co-trimoxazole (in HIV patients) and with proguanil plus
artesunate in healthy adults did not produce any changes in
atovaquone pharmacokinetics.
Clinical Use
Pn. jirovecii pneumonia; alternative therapy for mild to moderate illness
(prophylaxis and treatment)
Prophylaxis and treatment of malaria in combination with proguanil
It has also been used in cerebral toxoplasmosis in AIDS
patients and in a few cases of human babesiosis.
Clinical Use
3-[4-(4-Chlorophenyl)-cyclohexyl]-2-hydroxy-1,4-naphthoquinone(Mepron) is a highly lipophilic, water-insolubleanalog of ubiquinone 6, an essential component of the mitochondrialelectron transport chain in microorganisms. Thestructural similarity between atovaquone and ubiquinonesuggests that the former may act as an antimetabolite for thelatter and thereby interfere with the function of electrontransport enzymes.
Atovaquone was originally developed as an antimalarialdrug, but Plasmodium falciparum was found to developa rapid tolerance to its action. More recently, the effectivenessof atovaquone against P. carinii was discovered. Itis a currently recommended alternative to trimethoprimsulfamethoxazole(TMP-SMX) for the treatment and prophylaxisof PCP in patients intolerant to this combination.Atovaquone was also shown to be effective in eradicatingT. gondii in preclinical animal studies.
The oral absorption of atovaquone is slow and incomplete,in part because of the low water solubility of the drug.Aqueous suspensions provide significantly better absorptionthan do tablets. Food, especially if it has a high fat content,increases atovaquone absorption. Significant enterohepaticrecycling of atovaquone occurs, and most (nearly 95%) ofthe drug is excreted unchanged in the feces. In vivo, atovaquoneis largely confined to the plasma, where it is extensivelyprotein bound ( 99.9%). The half-life of the drugranges from 62 to 80 hours. The primary side effect is gastrointestinalintolerance.
Side Effects
Most clinical trials of atovaquone alone have involved patients with AIDS in whom adverse effects are often difficult to detect; however, more than 20% reported fever, nausea, diarrhea and rashes. There were limited changes in hepatocellular function. In malaria, in combination with proguanil, there are few reported side effects.
Veterinary Drugs and Treatments
Atovaquone (with azithromycin) appears effective in treating dogs with Babesia gibsoni (Asian genotype) infections, particularly in dogs not immunosuppressed or splenectomized. Atovaquone may be of benefit for treating pneumocystosis in dogs, but it is considered second line therapy after potentiated sulfonamides. Atovaquone (with azithromycin) may be of benefit in treating Cytauxzoon felis infections in cats (research is in progress at the time of writing).
Drug interactions
Potentially hazardous interactions with other drugs
Antibacterials: avoid with rifabutin, concentration
of both drugs reduced; avoid with rifampicin,
concentration reduced and rifampicin concentration
increased; concentration reduced by tetracycline.
Antivirals: concentration reduced by efavirenz -
avoid; concentration of indinavir possibly reduced;
concentration of zidovudine increased.
Metoclopramide: significant reduction in plasma
atovaquone levels.
Metabolism
Some evidence suggests limited metabolism (although no metabolites have been identified).
Metabolism
Atovaquone is poorly absorbed from the GI tract because of its poor water solubility and high fat solubility, but the absorption can be significantly increased if taken with a fat-rich meal. The drug is highly bound to plasma protein (94%) and does not enter the CNS in significant quantities. It is not significantly metabolized in humans and is exclusively eliminated in feces via the bile.
Properties of Atovaquone
| Melting point: | 216-2190C |
| Boiling point: | 535.0±50.0 °C(Predicted) |
| Density | 1.349±0.06 g/cm3(Predicted) |
| storage temp. | 2-8°C |
| solubility | DMSO: >10mg/mL |
| form | powder |
| pka | 5.01±0.10(Predicted) |
| color | yellow |
| λmax | 494nm(aq. Buffer)(lit.) |
| Merck | 14,866 |
| CAS DataBase Reference | 95233-18-4(CAS DataBase Reference) |
Safety information for Atovaquone
| Signal word | Warning |
| Pictogram(s) |
 Exclamation Mark Irritant GHS07 |
| GHS Hazard Statements |
H413:Hazardous to the aquatic environment, long-term hazard |
| Precautionary Statement Codes |
P273:Avoid release to the environment. |
Computed Descriptors for Atovaquone
| InChIKey | KUCQYCKVKVOKAY-CTYIDZIISA-N |
Atovaquone manufacturer
SRINI PHARMACEUTICALS PVT LTD
New Products
4,4-Difluoropiperidine hydrochloride tert-butyl 9-methoxy-3-azaspiro[5.5]undecane-3-carboxylate Indole Methyl Resin N-Isopropylurea N,N-Dicyclohexylcarbodiimide(DCC) MELDRUMS ACID 5-METHYLISOXAZOLE-4-CARBOXYLIC ACID Magnessium Bis glycinate Zinc ascorbate 1-bromo-2-butyne 2-acetamidophenol 9(10H)-anthracenone Erythrosin B, 4-Piperidinopiperidine 2-((4-morpholinophenylamino) (methylthio) methylene) malononitrile 2,4-dihydroxybenzaldehyde 3-(4-morpholinophenylamino)-5-amino-1H-pyrazole-4-carbonitrile Methyl 2-methylquinoline-6-carboxylate 2,6-dichloro-4-nitropyridine 4-Bromo-2-chlorobenzonitrile 2-(benzylamino)acetic acid hydrochloride 4-(tert-Butoxycarbonylamino)but- 2-ynoic acid 3,4-dihydro-2H-benzo[b][1,4]dioxepine 1-Phenyl-1-cycloprppanecarboxylicacidRelated products of tetrahydrofuran

You may like
-
 Atovaquone 98%View Details
Atovaquone 98%View Details -
 Atovaquone 98%View Details
Atovaquone 98%View Details -
 Atovaquone 98%View Details
Atovaquone 98%View Details -
 Atovaquone 98.00% CAS 95233-18-4View Details
Atovaquone 98.00% CAS 95233-18-4View Details
95233-18-4 -
 Atovaquone USP Powder, 25KgView Details
Atovaquone USP Powder, 25KgView Details
95233-18-4 -
 Atovaquone Api powder, USPView Details
Atovaquone Api powder, USPView Details
95233-18-4 -
 Atovaquone api powder, 1 Kg, Non prescriptionView Details
Atovaquone api powder, 1 Kg, Non prescriptionView Details
95233-18-4 -
 AtovaquoneView Details
AtovaquoneView Details
95233-18-4
