95233-18-4
Product Name:
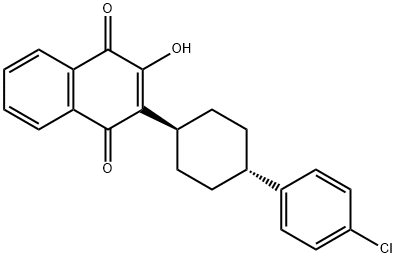
Atovaquone
Formula:
C22H19ClO3
Synonyms:
Atovaquone;Mepron;trans-2-[4-(4-Chlorophenyl)cyclohexyl]-3-hydroxy-1,4-naphthalenedione
Inquiry
CHEMICAL AND PHYSICAL PROPERTIES
| Color/Form | Crystals from acetonitrile |
|---|---|
| Melting Point | 216-219 °C |
| Solubility | Practically insoluble |
| LogP | 5.8 |
SAFETY INFORMATION
| Signal word | Warning |
|---|---|
| Pictogram(s) |
 Exclamation Mark Irritant GHS07 |
| GHS Hazard Statements |
H413:Hazardous to the aquatic environment, long-term hazard |
| Precautionary Statement Codes |
P273:Avoid release to the environment. |
COMPUTED DESCRIPTORS
| Molecular Weight | 366.8 g/mol |
|---|---|
| XLogP3 | 5.2 |
| Hydrogen Bond Donor Count | 1 |
| Hydrogen Bond Acceptor Count | 3 |
| Rotatable Bond Count | 2 |
| Exact Mass | 366.1022722 g/mol |
| Monoisotopic Mass | 366.1022722 g/mol |
| Topological Polar Surface Area | 54.4 Ų |
| Heavy Atom Count | 26 |
| Formal Charge | 0 |
| Complexity | 595 |
| Isotope Atom Count | 0 |
| Defined Atom Stereocenter Count | 0 |
| Undefined Atom Stereocenter Count | 0 |
| Defined Bond Stereocenter Count | 0 |
| Undefined Bond Stereocenter Count | 0 |
| Covalently-Bonded Unit Count | 1 |
| Compound Is Canonicalized | Yes |
PRODUCT INTRODUCTION
description
Atovaquone is a naphthoquinone compound having a 4-(4-chlorophenyl)cyclohexyl group at the 2-position and a hydroxy substituent at the 3-position. It has a role as an antimalarial, an antifungal agent, an EC 1.3.5.2 [dihydroorotate dehydrogenase (quinone)] inhibitor, an EC 1.6.5.3 [NADH:ubiquinone reductase (H(+)-translocating)] inhibitor and an EC 1.10.2.2 (quinol--cytochrome-c reductase) inhibitor. It is a member of monochlorobenzenes and a hydroxy-1,4-naphthoquinone.