Armodafinil
Synonym(s):(R)-Modafinil; 2-[(R)-(Diphenylmethyl)sulfinyl]-acetamide, CEP 10952, CRL 40982, Nuvigil
- CAS NO.:112111-43-0
- Empirical Formula: C15H15NO2S
- Molecular Weight: 273.35
- MDL number: MFCD09841055
- SAFETY DATA SHEET (SDS)
- Update Date: 2023-06-08 09:03:01
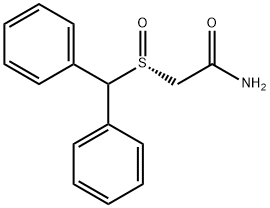
What is Armodafinil?
Absorption
Tmax is 2 hours when fasted and can be delayed approximately 2-4 hours by food, potentially affecting the onset of action.
Description
Armodafinil, an α1-adrenoceptor agonist, was launched for the
oral treatment of excessive sleepiness associated with narcolepsy, SWSD, and
obstructive sleep apnea/hypopnea syndrome (OSA). It is the R-enantiomer
of modafinil, which is a previously marketed wake-promoting agent. The key
differentiator for armodafinil is its longer pharmacokinetic half-life as
compared with the S-enantiomer (10-14 hvs.3-4h). At therapeutic
concentrations, armodafinil does not bind to most of the potentially
relevant receptors for sleep/wake regulation (e.g., serotonin, dopamine,
and adenosine receptors) or transporters of neurotransmitters or enzymes
involved in sleep/wake regulation (e.g., serotonin, norepinephrine, and
phosphodiesterase VI transporters). Both armodafinil and modafinil block
dopamine reuptake by binding to the dopamine transporter and increasing
dopamine concentrations in certain regions of the brain. However, dopamine
receptor antagonists (e.g., haloperidol) and dopamine synthesis inhibitors
(e.g., α-methyl-p-tyrosine) do not block modafinil’s action.
In addition to its wake-promoting effects and ability to increase locomotor activity in animals, modafinil produces psychoactive and euphoric effects, alterations in mood, perception, thinking, and feelings typical of other CNS stimulants in humans. Modafinil was also partially discriminated as stimulant-like. However, the potential for abuse and dependency appears to be lower for modafinil than amphetamine-like stimulants.The most common adverse
events associated with armodafinil included headache, nausea, dizziness,
and insomnia.
Chemical properties
White Solid
Originator
Cephalon (US)
The Uses of Armodafinil
Used for treatment of excessive sleepiness, a1-adrenoceptor agonist
The Uses of Armodafinil
Used for treatment of excessive sleepiness, α1-adrenoceptor agonist.
The Uses of Armodafinil
analeptic
Indications
Investigated for use/treatment in sleep disorders, obstructive sleep apnea, schizophrenia and schizoaffective disorders, depression, and bipolar disorders.
Background
Armodafinil is the enantiopure of the wakefulness-promoting agent modafinil (Provigil), and is indicated to improve wakefulness in adult patients with excessive sleepiness associated with obstructive sleep apnea (OSA), narcolepsy, or shift work disorder (SWD). Research has shown that armodafinil significantly improves driving simulator performance in patients with SWD. Armodafinil consists of the (?)-R-enantiomer of the racemic modafinil. Armodafinil is produced by the pharmaceutical company Cephalon Inc. (a wholly owned subsidiary of Teva Pharmaceutical Industries Ltd.) and was approved by the U.S. Food and Drug Administration (FDA) in June 2007.
Definition
ChEBI: A 2-[(diphenylmethyl)sulfinyl]acetamide that has R configuration at the sulfur atom. Like its racemate, modafinil, it is used for the treatment of sleeping disorders such as narcolepsy, obstructive sleep apnoea, and shift-work sleep disord r. Peak concentration in the blood later occurs later following administration than with modafinil, so it is thought that armodafinil may be more effective than modafinil in treating people with excessive daytime sleepiness.
brand name
Nuvigil
Synthesis
Armodafinil can be obtained starting from (+)-2( diphenylmethylsulfinyl)acetic acid via optical resolution using S-(-)-amethylbenzylamine, followed by esterification to the methyl ester and subsequent amidation with ammonium hydroxide. It can also be derived enantioselectively from 2-(diphenylmethylthio)acetamide via asymmetric oxidation with cumene hydroperoxide and titanium isopropoxide-S,Sdiethyl tartrate complex.
Metabolism
In vitro and in vivo data show that armodafinil undergoes hydrolytic deamidation, S-oxidation, and aromatic ring hydroxylation, with subsequent glucuronide conjugation of the hydroxylated products. Amide hydrolysis is the single most prominent metabolic pathway, with sulfone formation by cytochrome P450 (CYP) 3A4/5 being next in importance. The other oxidative products are formed too slowly in vitro to enable identification of the enzyme(s) responsible. Only two metabolites reach appreciable concentrations in plasma (i.e., R-modafinil acid and modafinil sulfone). Data specific to armodafinil disposition are not available.
Properties of Armodafinil
| Melting point: | 156-1580C |
| Boiling point: | 559.1±50.0 °C(Predicted) |
| Density | 1.283 |
| storage temp. | Controlled Substance, -20°C Freezer |
| solubility | DMSO: ≥16mg/mL |
| form | powder |
| pka | 14.88±0.40(Predicted) |
| color | white to tan |
| optical activity | [α]/D -15 to -20° in methanol (C=1) |
Safety information for Armodafinil
| Signal word | Warning |
| Pictogram(s) |
 Exclamation Mark Irritant GHS07  Health Hazard GHS08 |
| GHS Hazard Statements |
H302:Acute toxicity,oral |
| Precautionary Statement Codes |
P202:Do not handle until all safety precautions have been read and understood. P264:Wash hands thoroughly after handling. P264:Wash skin thouroughly after handling. P270:Do not eat, drink or smoke when using this product. P280:Wear protective gloves/protective clothing/eye protection/face protection. P301+P312:IF SWALLOWED: call a POISON CENTER or doctor/physician IF you feel unwell. P308+P313:IF exposed or concerned: Get medical advice/attention. |
Computed Descriptors for Armodafinil
Armodafinil manufacturer
New Products
(S)-3-Aminobutanenitrile hydrochloride 4-Methylphenylacetic acid N-Boc-D-alaninol N-BOC-D/L-ALANINOL Tert-butyl bis(2-chloroethyl)carbamate 3-Morpholino-1-(4-nitrophenyl)-5,6-dihydropyridin- 2(1H)-one Furan-2,5-Dicarboxylic Acid Tropic acid 1-Bromo-3,5-Di-Tert-Butylbenzene S-2-CHLORO PROPIONIC ACID ETHYL ISOCYANOACETATE 2-Bromo-1,3-Bis(Dimethylamino)Trimethinium Hexafluorophosphate 4-IODO BENZOIC ACID 3-NITRO-2-METHYL ANILINE 1-(2,4-DICHLOROPHENYL) ETHANAMINE (2-Hydroxyphenyl)acetonitrile 4-Bromopyrazole 2-(Cyanocyclohexyl)acetic acid 4-methoxy-3,5-dinitropyridine 1-(4-(aminomethyl)benzyl)urea hydrochloride 2-aminopropyl benzoate hydrochloride diethyl 2-(2-((tertbutoxycarbonyl)amino) ethyl)malonate tert-butyl 4- (ureidomethyl)benzylcarbamate Ethyl-2-chloro((4-methoxyphenyl)hydrazono)acetateRelated products of tetrahydrofuran

![2-[(Diphenylmethyl)sulfonyl] Acetamide](https://img.chemicalbook.in/CAS/GIF/118779-53-6.gif)
![2-[(Diphenylmethyl)thio]acetamide](https://img.chemicalbook.in/CAS/GIF/68524-30-1.gif)
You may like
-
 2033-24-1 98%View Details
2033-24-1 98%View Details
2033-24-1 -
 42831-50-5 5-METHYLISOXAZOLE-4-CARBOXYLIC ACID 98%View Details
42831-50-5 5-METHYLISOXAZOLE-4-CARBOXYLIC ACID 98%View Details
42831-50-5 -
 1975-50-4 98%View Details
1975-50-4 98%View Details
1975-50-4 -
 2-HYDROXY BENZYL ALCOHOL 98%View Details
2-HYDROXY BENZYL ALCOHOL 98%View Details
90-01-7 -
 2-Chloro-1,3-Bis(Dimethylamino)Trimethinium Hexafluorophosphate 221615-75-4 98%View Details
2-Chloro-1,3-Bis(Dimethylamino)Trimethinium Hexafluorophosphate 221615-75-4 98%View Details
221615-75-4 -
 61397-56-6 CIS BROMO BENZOATE 98%View Details
61397-56-6 CIS BROMO BENZOATE 98%View Details
61397-56-6 -
 14714-50-2 (2-Hydroxyphenyl)acetonitrile 98+View Details
14714-50-2 (2-Hydroxyphenyl)acetonitrile 98+View Details
14714-50-2 -
 118753-70-1 98+View Details
118753-70-1 98+View Details
118753-70-1