Arecoline hydrobromide
Synonym(s):1-Methyl-1,2,5,6-tetrahydro-3-pyridinecarboxylic acid methyl ester hydrobromide;Arecaidine methyl ester hydrobromide;Methyl 1,2,5,6-tetrahydro-1-methyl-3-pyridinecarboxylate hydrobromide;Methyl 1-methyl-1,2,5,6-tetrahydronicotinate hydrobromide;Taeniolin
- CAS NO.:300-08-3
- Empirical Formula: C8H14BrNO2
- Molecular Weight: 236.11
- MDL number: MFCD00039041
- EINECS: 206-087-3
- SAFETY DATA SHEET (SDS)
- Update Date: 2025-12-15 14:23:52
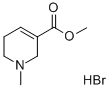
What is Arecoline hydrobromide ?
Description
Arecoline (CAS 300-8-3) is a covalent inhibitor of ACAT1 which binds to and disrupts only ACAT1 tetramers (IC50=11.1 μM). ACAT2 and DLAT are not inhibited. Tyrosine407 phosphorylation activates mitochondrial acetyl-CoA acetyltransferase 1 (ACAT1) by stabilizing its tetramer. This is believed to be the mechanisms by which ACAT1 is “hijacked” and contributes to the Warburg effect in cancer.? Treatment of xenograft nude mice with Arecoline resulted in a dose-dependent reduction in tumor mass.1?Agonist at muscarinic acetylcholine receptors M1 – M5 (EC50?in the range of 7-410 nM).2?May be effective in dementia.3
Chemical properties
WHITE FINE POWDER
The Uses of Arecoline hydrobromide
Arecoline is a muscarinic acetylcholine receptor agonist. Arecoline induces ADP ribosylation of histones and chromatid relaxation in spleen and bone marrow cells. In a study, a controlled trial involving 122 dogs experimentally infected with Echinococcus
The Uses of Arecoline hydrobromide
A cholinergic alkaloid from seeds of the betel nut palm Areca catechu. Anthelmintic (Cestodes); cathartic.
What are the applications of Application
Arecoline hydrobromide is an alkaloid originally extracted from betal nut palm seed
Preparation
According to a historical 1911 version of the German Pharmacopoeia, arecoline (as its hydrobromide; Arecoline hydrobromide) was produced from areca nuts using extraction with acidified water followed by several clean-up steps. The first industrial-scale extraction, reported in 1927, was based on the extraction of arecoline with diethyl ether. There are various approaches for the synthetic production of arecoline starting from nicotinic acid and iodomethane; methylamine hydrochloride, formaldehyde and acetaldehyde; or ethyl acrylate and methylamine. The most modern approach involves nicotinic acid methyl ester and methyl iodide.
Arecoline salts such as arecoline hydrochloride or arecoline hydrobromide may be obtained by dissolving arecoline in alcohol of low relative molecular mass (such as methanol, ethanol, isopropanol, butanol, or amyl alcohol) and adding sufficient amounts of acid (hydrochloric or bromic acid) to give a weakly acidic solution. The crystallized salts may be separated from the alcohol by filtration.
General Description
Arecoline is an alkaloid obtained from theseeds of the betel nut (Areca catechu). For many years, nativesof the East Indies have consumed the betel nut as asource of a euphoria-creating substance.
Safety Profile
Poison by parenteral,subcutaneous, and intravenous routes. When heated todecomposition it emits very toxic fumes of HBr and NOx.
Toxicology
Eighty rats were randomly divided into four groups: a high-dose group (1000 mg/kg), a medium-dose group (200 mg/kg), a low-dose group (100mg/kg), and a blank control group. The doses were administered daily via gastric lavage for 14 consecutive days. There were no significant differences in the low-dose Arecoline hydrobromide (Ah) group compared to the control group (P>0.05) with regard to body weight, organ coefficients, hematological parameters, and histopathological changes. In this study, the rats in the high-dose group exhibited clear inhibitory effects on blood biochemical parameters, including ALT, TP, and BUN levels. It was demonstrated that the toxicity reaction was intensified at the higher dosage, and clinical dosing should occur within a safe dosage range. With a continuous increase in the Ah dose, organ damage was aggravated, with different degrees of congestion, degeneration, and necrosis observed. The results accurately reflected the liver and kidney changes, whereas the results for the changes in the lung were poor. Hence, a long-term, continuous high dose of Ah was toxic[4].
References
1) Fan et al. (2016), Tetrameric Acetyl-CoA Acetyltransferase 1 is Important for Tumor Growth; Mol. Cell,?64?859
2) Heinrich et al. (2009), Pharmacological comparison of muscarinic ligands: historical versus more recent muscarinic M1-preferring receptor agonists; Eur. J. Pharmacol., 605?53
3) Christie et al. (1981), Physostigmine and arecoline: effects of intravenous infusions in Alzheimer presenile dementia; Br. J. Psychiatry, 138 46
4) Xiaojuan Wei. “Evaluation of arecoline hydrobromide toxicity after a 14-day repeated oral administration in Wistar rats.” PLoS ONE (2015): e0120165.
Properties of Arecoline hydrobromide
| Melting point: | 171-175 °C |
| storage temp. | Inert atmosphere,Room Temperature |
| solubility | H2O: 0.1 g/mL, clear, colorless |
| pka | 6.84(at 25℃) |
| form | Powder |
| color | White to light yellow |
| Merck | 14,778 |
| BRN | 3914826 |
| Stability: | Stable for 2 years from date of purchase as supplied. Solutions are not stable – make fresh daily. |
| InChI | InChI=1S/C8H13NO2.BrH/c1-9-5-3-4-7(6-9)8(10)11-2;/h4H,3,5-6H2,1-2H3;1H |
| CAS DataBase Reference | 300-08-3(CAS DataBase Reference) |
| EPA Substance Registry System | Arecoline hydrobromide (300-08-3) |
Safety information for Arecoline hydrobromide
| Signal word | Warning |
| Pictogram(s) |
 Exclamation Mark Irritant GHS07 |
| GHS Hazard Statements |
H302:Acute toxicity,oral |
| Precautionary Statement Codes |
P264:Wash hands thoroughly after handling. P264:Wash skin thouroughly after handling. P270:Do not eat, drink or smoke when using this product. P301+P312:IF SWALLOWED: call a POISON CENTER or doctor/physician IF you feel unwell. P501:Dispose of contents/container to..… |
Computed Descriptors for Arecoline hydrobromide
| InChIKey | AXOJRQLKMVSHHZ-UHFFFAOYSA-N |
| SMILES | C1(C(=O)OC)=CCCN(C)C1.Br |
Arecoline hydrobromide manufacturer
JSK Chemicals
New Products
Indole Methyl Resin tert-butyl 9-methoxy-3-azaspiro[5.5]undecane-3-carboxylate Boc-His(Boc)-OH 2-CTC Resin 4-Chloro-7-tosy1-7Hpyrrolo[2,3-d]pyrimidine 5,7-Dibromo-1H-indole 2,5-dichloro-N-hydroxy-4,6-dimethylpyridine-3-carboximidamide 2,2-Dimethoxy-7-azaspiro[3.5]nonane hydrochloride 4-chloromethyl-5-methyl-1,3-dioxol-2-one (DMDO-Cl) R-2-BENZYLOXY PROPIONIC ACID 1,1’-CARBONYLDIIMIDAZOLE 1,1’-CARBONYLDI (1,2-4 TRIAZOLE) N-METHYL INDAZOLE-3-CARBOXYLIC ACID 4-((2-hydroxyethyl)thio)benzoic acid 1-(TERT-BUTOXYCARBONYL)-2-PYRROLIDINONE Methyl 6-methylnicotinate 3-Pyridineacrylic acid tert-Butyl carbazate TETRAHYDRO-2H-PYRAN-3-OL 2-((4-morpholinophenylamino) (methylthio) methylene) malononitrile 3-(4-morpholinophenylamino)-5-amino-1H-pyrazole-4-carbonitrile 2,4-dihydroxybenzaldehyde 1,3-Diethyl-1,3-Diphenylurea Methyl 2-methylquinoline-6-carboxylateRelated products of tetrahydrofuran



You may like
-
 300-08-3 Arecoline hydrobromide, 98% 99%View Details
300-08-3 Arecoline hydrobromide, 98% 99%View Details
300-08-3 -
 Arecoline hydrobromide 95% CAS 300-08-3View Details
Arecoline hydrobromide 95% CAS 300-08-3View Details
300-08-3 -
 Arecoline hydrobromide, for HPLC >98% CAS 300-08-3View Details
Arecoline hydrobromide, for HPLC >98% CAS 300-08-3View Details
300-08-3 -
 Arecoline HBr 98% (HPLC) CAS 300-08-3View Details
Arecoline HBr 98% (HPLC) CAS 300-08-3View Details
300-08-3 -
 Arecoline Hydrobromide CAS 300-08-3View Details
Arecoline Hydrobromide CAS 300-08-3View Details
300-08-3 -
 Arecoline hydrobromide CAS 300-08-3View Details
Arecoline hydrobromide CAS 300-08-3View Details
300-08-3 -
 Pyridine 99.5% HPLC /UV SpectroscopyView Details
Pyridine 99.5% HPLC /UV SpectroscopyView Details
110-86-1 -
 Thiourea 99% ARView Details
Thiourea 99% ARView Details
62-56-6
