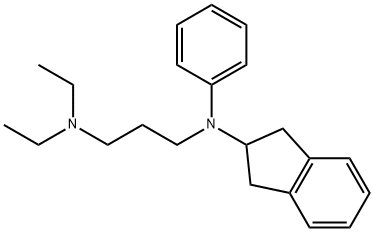
APRINDINE HCL
Synonym(s):N-(2,3-Dihydro-1H-inden-2-yl)-N′,N′-diethyl-N-phenyl-1,3-propanediamine hydrochloride;Amidonal
- CAS NO.:33237-74-0
- Empirical Formula: C22H31ClN2
- Molecular Weight: 358.95
- MDL number: MFCD01698392
- EINECS: 251-418-7
- SAFETY DATA SHEET (SDS)
- Update Date: 2024-10-28 23:16:16
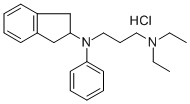
What is APRINDINE HCL?
Originator
Amidonal,Madaus,W. Germany,1976
The Uses of APRINDINE HCL
Aprindine is a long-acting antiarrhythmic agent, effective when administered orally or intravenously in the treatment of ventricular arrhythmias of varying etiologies.
What are the applications of Application
Aprindine hydrochloride is an hERG channel blocker
Definition
ChEBI: Aprindine hydrochloride is a member of indanes.
Manufacturing Process
104.6 g (0.5 mol) N-phenyl-2-aminoindane and 2.5 liters benzene are
introduced into a reaction vessel of 5 liters, under an atmosphere of nitrogen.
37 g (0.95 mol) sodium amide are added and the mixture is stirred during 3
hours at room temperature.
119.7 g (0.8 mol) of γ-chloropropyl diethylamine are then quickly added. After
agitation during 1 hour at room temperature, the reaction mixture is refluxed
and stirred under nitrogen during 21 hours. The mixture is then allowed to
cool and poured onto ice. The obtained aqueous phase is extracted by means
of 500 cm3 of benzene. The benzene extract is washed two times with 200
cm3 of water and the benzene is then evaporated.
The residue is treated with 500 cm3 of hydrochloric acid (2 N). The obtained
solution is evaporated to dryness and the oily residue is recrystallized from
ethanol. 176.9 g (yield 89.4%) of dihydrochloride of N-phenyl-Ndiethylaminopropyl-
2-aminoindane are obtained, MP 208° to 210°C.
The dihydrochloride is converted into monohydrochloride by dissolving 26.36 g
(0.066 mol) of dihydrochloride into 158 cm3 of water, adding drop by drop a
suitable amount (0.066 mol) of caustic soda (1 N), evaporating the aqueous
solution to dryness, drying by means of benzene, filtering the formed sodium
chloride (3.8 g) and crystallizing the cooled obtained benzene solution. 22.6 g
(95%) of monohydrochloride are obtained, MP 120° to 121°C.
brand name
Fibocil (Lilly).
Therapeutic Function
Antiarrhythmic
Properties of APRINDINE HCL
| Melting point: | 120-121° |
| storage temp. | 2-8°C |
| solubility | H2O: >10mg/mL |
| form | solid |
| color | Light Brown to Brown |
| Stability: | Hygroscopic |
Safety information for APRINDINE HCL
| Signal word | Warning |
| Pictogram(s) |
 Exclamation Mark Irritant GHS07 |
| GHS Hazard Statements |
H302:Acute toxicity,oral H315:Skin corrosion/irritation H319:Serious eye damage/eye irritation H335:Specific target organ toxicity, single exposure;Respiratory tract irritation |
| Precautionary Statement Codes |
P261:Avoid breathing dust/fume/gas/mist/vapours/spray. P264:Wash hands thoroughly after handling. P264:Wash skin thouroughly after handling. P270:Do not eat, drink or smoke when using this product. P301+P312:IF SWALLOWED: call a POISON CENTER or doctor/physician IF you feel unwell. P302+P352:IF ON SKIN: wash with plenty of soap and water. P305+P351+P338:IF IN EYES: Rinse cautiously with water for several minutes. Remove contact lenses, if present and easy to do. Continuerinsing. |
Computed Descriptors for APRINDINE HCL
New Products
Tert-butyl bis(2-chloroethyl)carbamate 4-Methylphenylacetic acid N-Boc-D-alaninol N-BOC-D/L-ALANINOL 3-Morpholino-1-(4-nitrophenyl)-5,6-dihydropyridin- 2(1H)-one Furan-2,5-Dicarboxylic Acid Tropic acid DIETHYL AMINOMALONATE HYDROCHLORIDE 1,1’-CARBONYLDIIMIDAZOLE R-2-BENZYLOXY PROPIONIC ACID 1,1’-CARBONYLDI (1,2-4 TRIAZOLE) N-METHYL INDAZOLE-3-CARBOXYLIC ACID (2-Hydroxyphenyl)acetonitrile 4-Bromopyrazole 5-BROMO-2CYANO PYRIDINE 5,6-Dimethoxyindanone 5-broMo-2-chloro-N-cyclopentylpyriMidin-4-aMine 2-(Cyanocyclohexyl)acetic acid 4-methoxy-3,5-dinitropyridine 2-aminopropyl benzoate hydrochloride 1-(4-(aminomethyl)benzyl)urea hydrochloride diethyl 2-(2-((tertbutoxycarbonyl)amino) ethyl)malonate tert-butyl 4- (ureidomethyl)benzylcarbamate Ethyl-2-chloro((4-methoxyphenyl)hydrazono)acetateRelated products of tetrahydrofuran

You may like
-
 Aprindine hydrochloride CAS 33237-74-0View Details
Aprindine hydrochloride CAS 33237-74-0View Details
33237-74-0 -
 1975-50-4 98%View Details
1975-50-4 98%View Details
1975-50-4 -
 2-HYDROXY BENZYL ALCOHOL 98%View Details
2-HYDROXY BENZYL ALCOHOL 98%View Details
90-01-7 -
 2-Chloro-1,3-Bis(Dimethylamino)Trimethinium Hexafluorophosphate 221615-75-4 98%View Details
2-Chloro-1,3-Bis(Dimethylamino)Trimethinium Hexafluorophosphate 221615-75-4 98%View Details
221615-75-4 -
 61397-56-6 CIS BROMO BENZOATE 98%View Details
61397-56-6 CIS BROMO BENZOATE 98%View Details
61397-56-6 -
 14714-50-2 (2-Hydroxyphenyl)acetonitrile 98+View Details
14714-50-2 (2-Hydroxyphenyl)acetonitrile 98+View Details
14714-50-2 -
 118753-70-1 98+View Details
118753-70-1 98+View Details
118753-70-1 -
 733039-20-8 5-broMo-2-chloro-N-cyclopentylpyriMidin-4-aMine 98+View Details
733039-20-8 5-broMo-2-chloro-N-cyclopentylpyriMidin-4-aMine 98+View Details
733039-20-8