Propylamine
Synonym(s):1-Aminopropane;Propylamine
- CAS NO.:107-10-8
- Empirical Formula: C3H9N
- Molecular Weight: 59.11
- MDL number: MFCD00008205
- EINECS: 203-462-3
- SAFETY DATA SHEET (SDS)
- Update Date: 2025-09-25 17:15:13
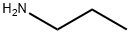
What is Propylamine?
Description
Propylamine is a colorless liquid that is slightly soluble in water. Specific gravity is 0.7, which is lighter than water. Propylamine is flammable, with a flammable range of 2%–10% in air. Boiling point is 120°F (48°C) and flash point is ?35°F (?37°C). Ignition temperature is 604°F (317°C). Vapor density is 2, which is heavier than air. In addition to being flammable, propylamine is corrosive and is a strong irritant to skin and tissue. The four-digit UN identification number is 1277. The NFPA 704 designation is health 3, flammability 3, and reactivity 0. It is shipped in glass bottles, cans, drums, and tank cars. The primary uses are as a chemical intermediate and as a lab reagent.
Chemical properties
n-Propylamine, also known as propylamine, is a colorless liquid with a pungent odor resembling ammonia. It has strong basic properties, leading to its easy formation of salts when combined with acids. Its reactivity is similar to that described for the other short chain aliphatic amines (Astel 1961).
The Uses of Propylamine
n-Propylamine is used as an intermediate inmany organic reactions.
The Uses of Propylamine
1-Propylamine is used in chemical analysis and to make other chemicals, used as a solvent in organic synthesis. And it is used as finishing agent of drugs, paints, rubber, fiber, textile and resin. Additionally, it can be used as petroleum additive and preservative.
Production Methods
There are several methods employed in the manufacture of propylamine. Typically, ammonia and propanol are reacted over a dehydration catalyst at high temperature and pressure. Ammonia, propanol, and hydrogen can also be reacted over a dehydrogenation catalyst such as metallic silver. Propylamine can also be synthesized from propionaldehyde and ammonia with a Raney nickel catalyst (Schweizer et al 1978).
Definition
ChEBI: Propylamine is a member of the class of alkylamines that is propane substituted by an amino group at C-1. It is a conjugate base of a propan-1-aminium.
Aroma threshold values
High strength odor; ammoniacal type; recommend smelling in a 0.10% solution or less.
General Description
A clear colorless liquid with an ammonia-like odor. Flash point -35°F. Less dense than water and soluble in water. Vapors are heavier than air. Produces toxic oxides of nitrogen during combustion. Used in chemical analysis and to make other chemicals.
Air & Water Reactions
Highly flammable. Slightly soluble in water.
Reactivity Profile
Colorless, alkaline liquid, very volatile (b. p. 48° C), moderately toxic, highly flammable. Dangerous fire hazard when exposed to heat, flame, sparks, or strong oxidizers. When heated to decomposition Propylamine emits toxic fumes of oxides of nitrogen. Incompatible with triethylaluminum, complex may explode on sublimation [Chini, P. et al., Chim. e Ind (Milan), 1962, 44, p. 1220].
Hazard
Highly flammable, dangerous fire risk, explosive limits in air 2–10%, use alcohol foam to extinguish. Strong irritant to skin and tissue.
Health Hazard
n-Propylamine is a strong irritant and a mod erately toxic substance. the toxic routes areinhalation, ingestion, and absorption throughthe skin. Contact of the liquid on the skincan cause burns and possibly skin sensitization. Irritation in the eyes from exposure tohigh concentrations can be severe. It is a respiratory tract irritant, similar to ethylamine.Inhalation of 2300 ppm of n-propylamine for4 hours caused labored breathing, hepatitis,and hepatocellular necrosis in rats. It is somewhat less toxic than ethylamine by oral anddermal routes.
LC50 value, inhalation (mice): 2500 mg/m3/2 hr
LD50 value, oral (rats): 570 mg/kg
LD50 value, skin (rabbits): 560 mg/kg.
Fire Hazard
Highly flammable liquid; flash point (closed
cup) -37°C (-35°F); vapor density 2.0
(air = 1), vapor can travel a considerable distance to a source of ignition and flash back;
autoignition temperature 318°C (604°F); fire extinguishing agent: dry chemical, CO2, or
“alcohol” foam; water should be used to keep
fire-exposed containers cool and to flush and
dilute the spill.
n-Propylamine forms explosive mixtures
in air; LEL and UEL values are 2.0% and
10.4% by volume in air, respectively. There
is no report of explosion associated with this
compound. It is expected to exhibit violent
reactions characteristic of lower aliphatic
primary amines .
Industrial uses
Propylamine is important as a chemical intermediate for rubber chemicals, dyestuffs, propyl isocyanate, textile and leather finishing resins and corrosion inhibitors. It is also used in the production of pharmaceuticals such as Prilocaine, pesticides including Profluralin and in petroleum additives. In 1976, 500 tons of propylamine were manufactured in the U.S. (HSDB 1988).
Safety Profile
Moderately toxic by inhalation, ingestion, and skin contact routes. A skin and severe eye irritant. Possibly a skin sensitizer. Very dangerous fire hazard when exposed to heat, flame, or oxidizers. Explosive in the form of vapor when exposed to heat or flame. To fight fire, use alcohol foam. When heated to decomposition it emits toxic fumes of NOx. Incompatible with triethynyl aluminum. See also AMINES.
Potential Exposure
Propylamine is used to make textile resins, drugs, pesticides, and other chemicals.
Metabolism
To date, there are several studies which indicate that propylamine may be
metabolized in many animal species, including man. When propylamine hydrochloride
was administered to humans orally, little of the parent compound was
recovered in the urine (Rechenberger 1940). It has also been reported that dogs are
capable of metabolizing propylamine (Bernhard 1938). McEwen et al (1966)
demonstrated that monoamine oxidase, purified from rabbit serum, was capable of
oxidizing propylamine, although less actively than substituted phenylethylamines
such as dopamine. Further characterization revealed that the protonated amine is
bound by an unprotonated enzyme group, that the enzyme active site is composed
of hydrophobic residues, and that interaction of the amine residues determines
maximal velocity and affinity constant (McEwen and Sober 1967). Oxidation of
the amine could be stimulated by the presence of aliphatic alcohols which
apparently bond in a tertiary complex with the enzyme and substrate, thereby
increasing the effectiveness of substrate binding.
Other investigators have provided evidence that propylamine may not be a
substrate for tissue monoamine oxidase. When given intraperitoneally to rats it had
no effect on the liver enzyme and little effect on activity in the brain (Valiev 1974).
Early work by Pugh and Quastel (1937) indicated that slices of rat brain did not
metabolize propylamine.
Shipping
UN1277 Propylamine, Hazard Class: 3; Labels: 3-Flammable liquid, 8-Corrosive material.
Purification Methods
Distil the amine from zinc dust, under reduced pressure, in an atmosphere of nitrogen. [Beilstein 4 IV 464.]
Incompatibilities
Vapors may form explosive mixture with air. Violent reaction on contact with oxidizers and mercury, strong acids; organic anhydrides; isocyanates, aldehydes, nitroparrafins, halogenated hydrocarbons; alcohols and many other compounds. Attacks many metals and alloys, especially those of copper. Aqueous solution is acidic and may attack glass.
Waste Disposal
Consult with environmental regulatory agencies for guidance on acceptable disposal practices. Generators of waste containing this contaminant (≥100 kg/mo) must conform with EPA regulations governing storage, transportation, treatment, and waste disposal.
Properties of Propylamine
| Melting point: | -83 °C (lit.) |
| Boiling point: | 48 °C (lit.) |
| Density | 0.719 g/mL at 25 °C (lit.) |
| vapor density | 2 (vs air) |
| vapor pressure | 4.79 psi ( 20 °C) |
| FEMA | 4237 | PROPYLAMINE |
| refractive index | n |
| Flash point: | −35 °F |
| storage temp. | Store below +30°C. |
| solubility | water: soluble0.4 part(lit.) |
| form | Liquid |
| pka | 10.6(at 20℃) |
| color | Clear colorless to pale yellow |
| Specific Gravity | 0.7173 |
| Odor | ammoniacal |
| PH | 12.6 (100g/l, H2O, 20℃) |
| explosive limit | 2.1-13.6%(V) |
| Odor Threshold | 0.061ppm |
| Water Solubility | soluble |
| Sensitive | Air Sensitive |
| Merck | 14,7843 |
| JECFA Number | 1580 |
| BRN | 1098243 |
| Exposure limits | No exposure limit has been set. Based on its
similarity to ethylamine in irritation and toxicity, a TLV-TWA of 10 ppm (~24 mg/m3)
should be appropriate.
. |
| Dielectric constant | 3.0(Ambient) |
| Stability: | Stable. Highly flammable. Note low flash point. Readily forms explosive mixtures with air. Incompatible with oxidising agents, strong acids. Store cool. |
| CAS DataBase Reference | 107-10-8(CAS DataBase Reference) |
| NIST Chemistry Reference | 1-Propanamine(107-10-8) |
| EPA Substance Registry System | Propylamine (107-10-8) |
Safety information for Propylamine
| Signal word | Danger |
| Pictogram(s) |
 Flame Flammables GHS02  Corrosion Corrosives GHS05  Skull and Crossbones Acute Toxicity GHS06 |
| GHS Hazard Statements |
H225:Flammable liquids H290:Corrosive to Metals H302:Acute toxicity,oral H314:Skin corrosion/irritation H335:Specific target organ toxicity, single exposure;Respiratory tract irritation |
| Precautionary Statement Codes |
P210:Keep away from heat/sparks/open flames/hot surfaces. — No smoking. P280:Wear protective gloves/protective clothing/eye protection/face protection. P301+P330+P331:IF SWALLOWED: Rinse mouth. Do NOT induce vomiting. P303+P361+P353:IF ON SKIN (or hair): Remove/Take off Immediately all contaminated clothing. Rinse SKIN with water/shower. |
Computed Descriptors for Propylamine
Propylamine manufacturer
New Products
4,4-Difluoropiperidine hydrochloride tert-butyl 9-methoxy-3-azaspiro[5.5]undecane-3-carboxylate Indole Methyl Resin N-Isopropylurea N,N-Dicyclohexylcarbodiimide(DCC) MELDRUMS ACID 5-METHYLISOXAZOLE-4-CARBOXYLIC ACID Magnessium Bis glycinate Zinc ascorbate 1-bromo-2-butyne 2-acetamidophenol 9(10H)-anthracenone Erythrosin B, 4-Piperidinopiperidine 2-((4-morpholinophenylamino) (methylthio) methylene) malononitrile 2,4-dihydroxybenzaldehyde 3-(4-morpholinophenylamino)-5-amino-1H-pyrazole-4-carbonitrile Methyl 2-methylquinoline-6-carboxylate 2,6-dichloro-4-nitropyridine 4-Bromo-2-chlorobenzonitrile 2-(benzylamino)acetic acid hydrochloride 4-(tert-Butoxycarbonylamino)but- 2-ynoic acid 3,4-dihydro-2H-benzo[b][1,4]dioxepine 1-Phenyl-1-cycloprppanecarboxylicacidRelated products of tetrahydrofuran
You may like
-
 n-Propylamine 99%View Details
n-Propylamine 99%View Details -
 n-Propylamine 98%View Details
n-Propylamine 98%View Details -
 1-Propylamine CAS 107-10-8View Details
1-Propylamine CAS 107-10-8View Details
107-10-8 -
 1-Propylamine CAS 107-10-8View Details
1-Propylamine CAS 107-10-8View Details
107-10-8 -
 Propylamine CAS 107-10-8View Details
Propylamine CAS 107-10-8View Details
107-10-8 -
 Propylamine, GR 99% CAS 107-10-8View Details
Propylamine, GR 99% CAS 107-10-8View Details
107-10-8 -
 Propylamine CAS 107-10-8View Details
Propylamine CAS 107-10-8View Details
107-10-8 -
 n-PROPYLAMINE For Synthesis CAS 107-10-8View Details
n-PROPYLAMINE For Synthesis CAS 107-10-8View Details
107-10-8