ANTIMONY(V) CHLORIDE
Synonym(s):Antimony pentachloride
- CAS NO.:7647-18-9
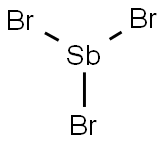
- Empirical Formula: Cl5Sb
- Molecular Weight: 299.02
- MDL number: MFCD00011213
- EINECS: 231-601-8
- SAFETY DATA SHEET (SDS)
- Update Date: 2024-12-18 14:15:32
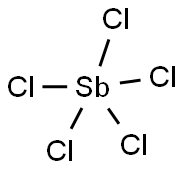
What is ANTIMONY(V) CHLORIDE?
Description
Antimony pentachloride is a noncombustible,colorless to reddish-yellow oily liquid with an offensive odor.Molecular weight= 299.05; Boiling point= 77℃ (decomposes); Freezing/Melting point= 3℃; Specific gravity(H2O:1)= 2.354 at 20℃; Liquid surface tension= (estimate) 50.015 N/m at 20℃; Latent heat of vaporization=1.60 3 105 J/kg; Heat of solution=2 4.925 3 105 J/kg;Vapor pressure: 0.84 mmHg at 20℃. Hazard Identification(based on NFPA-704 M Rating System): (solid or liquid)Health 3, Flammability 0, Reactivity 1. Decomposes on contact with water.
Chemical properties
colourless to light yellow oily liquid
Chemical properties
Antimony pentachloride is a noncombustible, colorless to reddish-yellow oily liquid with an offensive odor.
Physical properties
Antimony pentachloride is prepared by passing chlorine gas into molten antimony trichloride:
SbCl3 + Cl2 →SbCl5
or by the reaction of the element with excess chlorine:
2 Sb + 5 Cl2 → 2 SbCl5.
The Uses of ANTIMONY(V) CHLORIDE
Antimony(V) chloride is used as a catalyst and an analytical reagent for testing alkaloids and cesium. It is also used as a polymerization catalyst as well as involved in the chlorination of organic compounds. It acts as a Lewis acid and a strong oxidizing agent.
The Uses of ANTIMONY(V) CHLORIDE
As catalyst when replacing a fluorine substituent with chlorine in organic compounds.
What are the applications of Application
Antimony(V) chloride is an intermediate in chemical synthesis; also a strong oxidizing agent
General Description
A reddish-yellow fuming liquid with a pungent odor. Fumes are irritating to the eyes and mucous membranes. Solidifies at 37°F. Corrosive to metals and tissue. Used to make other chemicals, and in chemical analysis.
Air & Water Reactions
Fumes in air to form hydrochloric acid. Reacts with water to yield heat and antimony pentaoxide (Sb2O5) and hydrochloric acid [Merck 11th ed. 1989].
Reactivity Profile
Acidic salts, such as ANTIMONY PENTACHLORIDE, are generally soluble in water. The resulting solutions contain moderate concentrations of hydrogen ions and have pH's of less than 7.0. They react as acids to neutralize bases. These neutralizations generate heat, but less or far less than is generated by neutralization of inorganic acids, inorganic oxoacids, and carboxylic acid. They usually do not react as either oxidizing agents or reducing agents but such behavior is not impossible. Many of these compounds catalyze organic reactions.
Hazard
Corrosive, fumes in moist air, reacts strongly with organics.
Health Hazard
Inhalation causes irritation of nose and throat. Contact of liquid with eyes or skin causes severe burns. Ingestion causes vomiting and severe burns of mouth and stomach. Overexposure by any route can cause bloody stools, slow pulse, low blood pressure, coma, convulsions, cardiac arrest.
Fire Hazard
Behavior in Fire: Irritating fumes of hydrogen chloride given off when water or foam is used to extinguish adjacent fire.
Safety Profile
Poison by ingestion. Corrosive. Mutation data reported. See ANTIMONY COMPOUNDS and ANTIMONYPII) CHLORIDE. When heated to decomposition it emits very toxic fumes of Cland Sb.
Potential Exposure
It is used in dyeing, coloring metals and in many organic chemical reactions as a catalyst.
First aid
If this chemical gets into the eyes, remove anycontact lenses at once and irrigate immediately for at least15 min, occasionally lifting upper and lower lids. Seekmedical attention immediately. If this chemical contactsthe skin, remove contaminated clothing and wash immediately with soap and water. Seek medical attention immediately. If this chemical has been inhaled, remove fromexposure, begin rescue breathing (using universal precautions, including resuscitation mask) if breathing hasstopped and CPR if heart action has stopped. Transferpromptly to a medical facility. When this chemical hasbeen swallowed, get medical attention. If victim is conscious, administer water or milk. Do not induce vomiting.Medical observation is recommended for 24°48 h afterbreathing overexposure, as pulmonary edema may bedelayed. As first aid for pulmonary edema, a doctor orauthorized paramedic may consider administering a corticosteroid spray.
storage
(1) Color Code—White: Corrosive or ContactHazard; Store separately in a corrosion-resistant location.(2) Color Code—Blue: Health Hazard/Poison: Store in asecure poison location. Prior to working with this chemicalyou should be trained on its proper handling and storage.Antimony pentachloride must be stored to avoid contactwith organic or combustible materials (such as wood,paper, and oil) since violent reactions occur. See incompatibilities. Store in tightly closed containers in a cool, wellventilated area away from water or moisture and heat.Sources of ignition, such as smoking and open flames, areprohibited where this chemical is used, handled, or storedin a manner that could create a potential fire or explosionhazard.
Shipping
UN1730 (liquid) Antimony pentachloride, liquid, Hazard class: 8; Labels: 8-Corrosive material. UN1731 (solution) Antimony pentachloride, solutions, Hazard class: 8; Labels: 8-Corrosive material.
Incompatibilities
Decomposes on contact with heat, acids, alkalis, ammonia, water or other forms of moisture producing fumes of hydrogen chloride and antimony. Decomposes above 77 C forming chlorine and antimony trichloride. Attacks many metals in the presence of moisture forming explosive hydrogen gas. Reacts with air forming corrosive vapors.
Waste Disposal
Encapsulate and transfer to an approve landfill. If chemically treated and neutralized, the chemical is amenable to biological treatment at municipal sewage treatment plant. Consult with environmental regulatory agencies for guidance on acceptable disposal practices. Generators of waste containing this contaminant (≥100 kg/ mo) must conform with EPA regulations governing storage, transportation, treatment, and waste disposal.
Properties of ANTIMONY(V) CHLORIDE
| Melting point: | 2.8 °C(lit.) |
| Boiling point: | 92 °C30 mm Hg(lit.) |
| Density | 2.36 g/mL at 25 °C(lit.) |
| vapor density | >10.2 (vs air) |
| vapor pressure | 0.13 psi ( 55 °C) |
| refractive index | 1.601 |
| Flash point: | 79°C/22mm |
| solubility | Soluble in HCl, chloroform, carbon tetrachlorideMiscible with chloroform, hydrochloric acid, tartaric acid, carbon disulfide and carbon tetrachloride. |
| form | Liquid |
| color | Red-yellow |
| Specific Gravity | 2.336 |
| Odor | offensive odor |
| Water Solubility | Soluble in water (react), chloroform, hydrochloric acid, and carbon tetrachloride. |
| Sensitive | Moisture Sensitive |
| Merck | 14,695 |
| Dielectric constant | 3.2200000000000002 |
| Stability: | Stable, but reacts with moisture and water. Releases highly toxic fumes if involved in a fire. |
| CAS DataBase Reference | 7647-18-9(CAS DataBase Reference) |
| EPA Substance Registry System | Antimony pentachloride (7647-18-9) |
Safety information for ANTIMONY(V) CHLORIDE
| Signal word | Danger |
| Pictogram(s) |
 Corrosion Corrosives GHS05  Environment GHS09 |
| GHS Hazard Statements |
H314:Skin corrosion/irritation H411:Hazardous to the aquatic environment, long-term hazard |
| Precautionary Statement Codes |
P260:Do not breathe dust/fume/gas/mist/vapours/spray. P273:Avoid release to the environment. P280:Wear protective gloves/protective clothing/eye protection/face protection. P303+P361+P353:IF ON SKIN (or hair): Remove/Take off Immediately all contaminated clothing. Rinse SKIN with water/shower. P305+P351+P338:IF IN EYES: Rinse cautiously with water for several minutes. Remove contact lenses, if present and easy to do. Continuerinsing. |
Computed Descriptors for ANTIMONY(V) CHLORIDE
New Products
(S)-3-Aminobutanenitrile hydrochloride 4-Methylphenylacetic acid N-Boc-D-alaninol N-BOC-D/L-ALANINOL Tert-butyl bis(2-chloroethyl)carbamate 3-Morpholino-1-(4-nitrophenyl)-5,6-dihydropyridin- 2(1H)-one Furan-2,5-Dicarboxylic Acid Tropic acid 1-Bromo-3,5-Di-Tert-Butylbenzene S-2-CHLORO PROPIONIC ACID ETHYL ISOCYANOACETATE 2-Bromo-1,3-Bis(Dimethylamino)Trimethinium Hexafluorophosphate 4-IODO BENZOIC ACID 3-NITRO-2-METHYL ANILINE 1-(2,4-DICHLOROPHENYL) ETHANAMINE (2-Hydroxyphenyl)acetonitrile 4-Bromopyrazole 2-(Cyanocyclohexyl)acetic acid 4-methoxy-3,5-dinitropyridine 1-(4-(aminomethyl)benzyl)urea hydrochloride 2-aminopropyl benzoate hydrochloride diethyl 2-(2-((tertbutoxycarbonyl)amino) ethyl)malonate tert-butyl 4- (ureidomethyl)benzylcarbamate Ethyl-2-chloro((4-methoxyphenyl)hydrazono)acetateRelated products of tetrahydrofuran




You may like
-
 Antimony(V) chloride, Ultra dry CAS 7647-18-9View Details
Antimony(V) chloride, Ultra dry CAS 7647-18-9View Details
7647-18-9 -
 Antimony(V) chloride, Ultra dry CAS 7647-18-9View Details
Antimony(V) chloride, Ultra dry CAS 7647-18-9View Details
7647-18-9 -
 Antimony(V) chloride CAS 7647-18-9View Details
Antimony(V) chloride CAS 7647-18-9View Details
7647-18-9 -
 Antimony(V) chloride CAS 7647-18-9View Details
Antimony(V) chloride CAS 7647-18-9View Details
7647-18-9 -
 Antimony(V) chloride CAS 7647-18-9View Details
Antimony(V) chloride CAS 7647-18-9View Details
7647-18-9 -
 Antimony(V) chloride CAS 7647-18-9View Details
Antimony(V) chloride CAS 7647-18-9View Details
7647-18-9 -
 antimony pentachloride CASView Details
antimony pentachloride CASView Details -
 Antimony(V) chloride, 99% CAS 7647-18-9View Details
Antimony(V) chloride, 99% CAS 7647-18-9View Details
7647-18-9