Graphite
Synonym(s):Graphene foil;Graphite;XG Leaf B 50 μm
- CAS NO.:7782-42-5
- Empirical Formula: C
- Molecular Weight: 12.01
- MDL number: MFCD00144065
- EINECS: 231-955-3
- SAFETY DATA SHEET (SDS)
- Update Date: 2025-12-17 09:50:40

What is Graphite?
Chemical properties
soft dark grey solid
Chemical properties
Graphite is crystallized carbon and usually appears as soft, black scales. There are two types of graph ite, natural and artificial (activated). Natural and synthetic graphite may be mixed with each other or contain other additives.
Occurrence
Graphite is usually found in metamorphic rocks as veins, lenses, and pockets and as thin laminae disseminated in gneisses, schists, and phyllites. Depending upon the mode of occurrence
and origin, it is graded into three forms: flake graphite found in metamorphosed rocks as vein
deposits, crystalline graphite found as fissure-filled veins, and cryptocrystalline graphite formed in metamorphosed coal beds. Natural graphite occurs in many parts of the world in
fair abundance and it has been used in various applications.
In nature, graphite is found
usually in association with feldspars, mica, quartz, pyroxene, rutile, pyrites, and apatite.
These impurities are associated with vein graphite. The impurities with amorphous graphite
are shale, slate, sandstone, quartz, and limestone. Graphite is found in almost every country,
but Ceylon, Madagascar, Mexico, western Germany, and Korea all possess particularly plentiful reserves. Major industrial producers of graphite are South Korea, the largest producer
in the world, followed by Austria.
The Uses of Graphite
For "lead" pencils, refractory crucibles, stove polish; as pigment, lubricant, graphite cement; for matches and explosives, commutator brushes, anodes, arc-lamp carbons, electroplating; polishing Compounds, rust and needle-paper; coating for cathode ray tubes; moderator in nuclear piles.
The Uses of Graphite
Graphite has been used alone to make refractory products for the lower blast furnace linings, and electrodes for steel and aluminum production. They are also commonly used in conjunction with other refractory raw materials. These materials are highly refractory nonwettable materials and are useful refractories in nonoxidizing environments. Carbon blacks are co
The Uses of Graphite
Similar to those of natural graphite in refractories and electrical products
The Uses of Graphite
High-temperature lubricant, crucible container for handling molten metals such as Mg, Al, Zn, Ga, Sb, and Bi
Definition
An allotrope of CARBON. Graphite is a good conductor of heat and electricity. The atoms are arranged in layers which cleave easily and graphite is used as a solid lubricant.
Preparation
Impervious graphite is manufactured by processing graphite at temperatures above 2000°C using Acheson furnaces, evacuating the pores, and impregnating with a phenolic resin. The impregnation seals the porosity.
What are the applications of Application
Flake graphite containing 80 to 85 wt.% C is used for crucible manufacture; 93 wt.% C and
above is preferred for the manufacture of lubricants, and graphite with 40 to 70 wt.% C is used
for foundry facings. Natural graphite, refined or otherwise pure, having a carbon content of not
less than 95%, is used in the manufacture of carbon rods for dry battery cells.
The utility of graphite is dependent largely upon its type, i.e., flake, lumpy, or amorphous.
The flake-type graphite is found to possess extremely low resistivity to electrical conductance. The electrical resistivity decreases with an increase in flaky particles. In addition, the
bulk density decreases progressively as the particles become more and more flaky. Because
of this property in flake graphite, it enjoys widespread use in the manufacture of carbon
electrodes, plates, and brushes required in the electrical industry and dry-cell batteries.
General Description
A mineral form of the element carbon. Hexagonal crystals or thin leaf-like layers. Steel-gray to black with a metallic luster and a greasy feel. An electrical conductor. Used for high-temperature crucibles, as a lubricant and in "lead" pencils.
Reactivity Profile
GRAPHITE is non-flammable in bulk form, but combustible. A reducing agent. Mixtures of graphite dust and air are explosive when ignited.Reacts violently with very strong oxidizing agents such as fluorine, chlorine dioxide, and potassium peroxide. Almost inert chemically when in bulk form. Keep away from ignition sources and oxidizing agents.
Health Hazard
Pure synthetic graphite acts as an inert or nuisance dust.
Flammability and Explosibility
Non flammable
Industrial uses
Graphite is a form of carbon. It was formerlyknown as black lead, and when first used forpencils was called Flanders’ stone. It is a naturalvariety of elemental carbon with a grayishblackcolor and a metallic tinge.
Carbon and graphite have been used inindustry for many years, primarily as electrodes,arc carbons, brush carbons, and bearings.In the last decade or so, development ofnew types and emergence of graphite fibers asa promising reinforcement for high-performancecomposites have significantly increasedthe versatility of this family of materials.
Potential Exposure
Natural graphite is used in foundry facings, steel making lubricants, refractories, crucibles, pencil “lead,” paints, pigments, and stove polish. Artificial graphite may be substituted for these uses with the excep tion of clay crucibles; other types of crucibles may be pro duced from artificial graphite. Additionally, it may be used as a high temperature lubricant or for electrodes. It is uti lized in the electrical industry in electrodes, brushes, con tacts, and electronic tube rectifier elements; as a constituent in lubricating oils and greases; to treat friction elements, such as brake linings; to prevent molds from sticking together; and in moderators in nuclear reactors. In addition, concerns have been expressed about synthetic graphite in fibrous form. Those exposed are involved in production of graphite fibers from pitch or acrylonitrile fibers and the manufacture and use of composites of plastics, metals, or ceramics reinforced with graphite fibers.
Shipping
UN1362 Carbon, activated, Hazard Class: 4.2; Labels: 4.2-Spontaneously combustible material, International.
Purification Methods
Treat graphite with hot 1:1 HCl. Then filter, wash and the dried powdered is heated in an evacuated quartz tube at 1000o until a high vacuum is obtained. Cool this and store it in an atmosphere of helium [Craig et al. J Phys Chem 60 1225 1956].
Incompatibilities
Graphite is a strong reducing agent and reacts violently with oxidizers, such as fluorine, chlorine trifluoride, and potassium peroxide. Forms an explosive mixture with air. May be spontaneously combustible in air.
Waste Disposal
Do not incinerate. Carbon (graphite) fibers are difficult to dispose of by incineration. Waste fibers should be packaged and disposed of in a land fill authorized for the disposal of special wastes of this nature, or as otherwise may be required by law.
Properties of Graphite
| Melting point: | 3652-3697 °C(lit.) |
| Boiling point: | 4830°C |
| Density | 2.2 g/mL at 25 °C |
| storage temp. | no restrictions. |
| solubility | insoluble in H2O |
| form | rod |
| color | black |
| Specific Gravity | 2.25 |
| PH | 5-6 (50g/l, H2O, 20℃)(slurry) |
| Resistivity | (Electrical resistivity: surface 0.06 Ω/sq, sheet 2.7 μΩ m) |
| Water Solubility | Insoluble in water. |
| Sensitive | Air & Light Sensitive |
| Crystal Structure | Hexagonal |
| Merck | 13,4554 |
| Exposure limits | ACGIH: TWA 2 mg/m3 OSHA: TWA 15 mg/m3; TWA 5 mg/m3 NIOSH: IDLH 1250 mg/m3; TWA 2.5 mg/m3 |
| Dielectric constant | 12.0(Ambient) |
| NIST Chemistry Reference | Graphite(7782-42-5) |
| EPA Substance Registry System | Graphite (7782-42-5) |
Safety information for Graphite
| Signal word | Danger |
| Pictogram(s) |
 Flame Flammables GHS02  Exclamation Mark Irritant GHS07 |
| GHS Hazard Statements |
H228:Flammable solids H319:Serious eye damage/eye irritation H335:Specific target organ toxicity, single exposure;Respiratory tract irritation |
| Precautionary Statement Codes |
P210:Keep away from heat/sparks/open flames/hot surfaces. — No smoking. P261:Avoid breathing dust/fume/gas/mist/vapours/spray. P280:Wear protective gloves/protective clothing/eye protection/face protection. P304+P340:IF INHALED: Remove victim to fresh air and Keep at rest in a position comfortable for breathing. P305+P351+P338:IF IN EYES: Rinse cautiously with water for several minutes. Remove contact lenses, if present and easy to do. Continuerinsing. P405:Store locked up. |
Computed Descriptors for Graphite
Graphite manufacturer
JSK Chemicals
New Products
4,4-Difluoropiperidine hydrochloride tert-butyl 9-methoxy-3-azaspiro[5.5]undecane-3-carboxylate Indole Methyl Resin N-Isopropylurea N,N-Dicyclohexylcarbodiimide(DCC) MELDRUMS ACID 5-METHYLISOXAZOLE-4-CARBOXYLIC ACID Magnessium Bis glycinate Zinc ascorbate 1-bromo-2-butyne 2-acetamidophenol 9(10H)-anthracenone Erythrosin B, 4-Piperidinopiperidine 2-((4-morpholinophenylamino) (methylthio) methylene) malononitrile 2,4-dihydroxybenzaldehyde 3-(4-morpholinophenylamino)-5-amino-1H-pyrazole-4-carbonitrile Methyl 2-methylquinoline-6-carboxylate 2,6-dichloro-4-nitropyridine 4-Bromo-2-chlorobenzonitrile 2-(benzylamino)acetic acid hydrochloride 4-(tert-Butoxycarbonylamino)but- 2-ynoic acid 3,4-dihydro-2H-benzo[b][1,4]dioxepine 1-Phenyl-1-cycloprppanecarboxylicacidRelated products of tetrahydrofuran
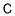







You may like
-
 Graphite rod CASView Details
Graphite rod CASView Details -
 Graphite rod CASView Details
Graphite rod CASView Details -
 Graphite electrode CASView Details
Graphite electrode CASView Details -
 Graphite electrode CASView Details
Graphite electrode CASView Details -
 Graphite electrode CASView Details
Graphite electrode CASView Details -
 Graphite electrode CASView Details
Graphite electrode CASView Details -
 Graphite rod CASView Details
Graphite rod CASView Details -
 Graphite rod CASView Details
Graphite rod CASView Details
