Antimony tribromide
- CAS NO.:7789-61-9
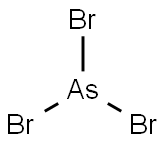
- Empirical Formula: Br3Sb
- Molecular Weight: 361.47
- MDL number: MFCD00016317
- EINECS: 232-179-8
- SAFETY DATA SHEET (SDS)
- Update Date: 2024-03-14 15:18:27
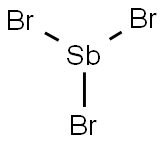
What is Antimony tribromide?
Description
Antimony tribromide is a nonflammable, colorless to yellow crystalline solid. Molecular weight= 361.51.Boiling point= 288℃ at 749 mmHg; Freezing/Meltingpoint=96.6℃; Specific gravity= 4.148 at 23℃; Criticaltemperature= 904.5℃; Critical pressure= 5.67 MN/m2;Heat of vaporization= 53.2 kJ/mol at 560℃; Vaporpressure= ,0.075 mmHg at 23℃. Hazard Identification(based on NFPA-704 M Rating System): Health 3,Flammability 0, Reactivity 1. Soluble in water, forms anacid.
Chemical properties
Yellow Crystalline Powder
Chemical properties
Antimony tribromide is a nonflammable, colorless to yellow crystalline solid dissolved in hydrobromic acid.
The Uses of Antimony tribromide
Analytical chemistry, mordant, manufacturing antimony salts.
The Uses of Antimony tribromide
Antimony(III) bromide is used in the production of other antimony compounds, in chemical analysis, as a mordant, and in dyeing. It can be added to polymers such as polyethylene as a fire retardant.
General Description
Antimony tribromide is the yellow crystalline solid dissolved in hydrobromic acid. Antimony tribromide is decomposed by water giving an antimony oxide and hydrobromic acid. Antimony tribromide is corrosive to metals and tissue. Antimony tribromide is used to make other antimony compounds, in chemical analysis, and in dyeing.
Air & Water Reactions
Water slowly hydrolyzes antimony tribromide to form antimony (III) oxide and hydrobromic acid. The dry powdered oxide ignites on heating in air [Mellor Vol. 9 425.1939].
Reactivity Profile
Acidic salts, such as ANTIMONY TRIBROMIDE, are generally soluble in water. The resulting solutions contain moderate concentrations of hydrogen ions and have pH's of less than 7.0. They react as acids to neutralize bases. These neutralizations generate heat, but less or far less than is generated by neutralization of inorganic acids, inorganic oxoacids, and carboxylic acid. They usually do not react as either oxidizing agents or reducing agents but such behavior is not impossible. Many of these compounds catalyze organic reactions.
Hazard
Toxic.
Health Hazard
TOXIC; inhalation, ingestion or contact (skin, eyes) with vapors, dusts or substance may cause severe injury, burns or death. Reaction with water or moist air will release toxic, corrosive or flammable gases. Reaction with water may generate much heat that will increase the concentration of fumes in the air. Fire will produce irritating, corrosive and/or toxic gases. Runoff from fire control or dilution water may be corrosive and/or toxic and cause pollution.
Fire Hazard
Non-combustible, substance itself does not burn but may decompose upon heating to produce corrosive and/or toxic fumes. Vapors may accumulate in confined areas (basement, tanks, hopper/tank cars etc.). Substance will react with water (some violently), releasing corrosive and/or toxic gases and runoff. Contact with metals may evolve flammable hydrogen gas. Containers may explode when heated or if contaminated with water.
Safety Profile
A poison. Corrosive to skin, eyes, and mucous membranes. Reaction with water liberates HBr and antimony trioxide. Can cause severe burns. See also ANTIMONY COMPOUNDS.
Potential Exposure
Antimony tribromide is used to make antimony salts; in dyeing, and analytical chemistry.
First aid
If this chemical gets into the eyes, remove anycontact lenses at once and irrigate immediately for at least15 min, occasionally lifting upper and lower lids. Seekmedical attention immediately. If this chemical contactsthe skin, remove contaminated clothing and wash immediately with soap and water. Seek medical attention immediately. If this chemical has been inhaled, remove fromexposure, begin rescue breathing (using universal precautions, including resuscitation mask) if breathing hasstopped and CPR if heart action has stopped. Transferpromptly to a medical facility. When this chemical hasbeen swallowed, get medical attention. If victim is conscious, administer water or milk. Do not induce vomiting.Medical observation is recommended for 24°48 h afterbreathing overexposure, as pulmonary edema may bedelayed. As first aid for pulmonary edema, a doctor orauthorized paramedic may consider administering acorticosteroid spray.
storage
Color Code—Blue: Health Hazard/Poison: Store ina secure poison location. Prior to working with this chemicalyou should be trained on its proper handling and storage.Antimony Tribromide must be stored to avoid contact withpotassium, sodium, and bases (such as sodium hydroxide,potassium hydroxide, and ammonium hydroxide) since violent reactions occur. Store in tightly closed containers in acool, well-ventilated area away from water or moisture andheat.
Shipping
UN3141 Antimony compounds, inorganic, liquid, n.o.s, Hazard Class: 6.1; Labels: 6.1-Poisonous materials, Technical Name Required.
Incompatibilities
Contact with water reacts, liberating antimony trioxide and hydrogen bromide. Dry trioxide material may explode if heated air. Heat forms toxic bromides. Antimony tribromide attacks various metals.
Waste Disposal
Encapsulate and bury at an approved chemical landfill. Unacceptable for disposal at sewage treatment plants. Consult with environmental regulatory agencies for guidance on acceptable disposal practices. Generators of waste containing this contaminant (≥100 kg/mo) must conform with EPA regulations governing storage, transportation, treatment, and waste disposal.
Properties of Antimony tribromide
| Melting point: | 97 °C |
| Boiling point: | 280 °C |
| Density | 4.15 g/mL at 25 °C(lit.) |
| vapor pressure | 1 mm Hg ( 94 °C) |
| refractive index | 1.74 |
| Flash point: | 280°C |
| solubility | Soluble in dilute hydrochloric acid, hydrobromic acid, carbon disulfide, alcohol, acetone, ammonia, benzene, chloroform. |
| form | Powder |
| color | White |
| Specific Gravity | 4.15 |
| Water Solubility | decomposed by H2O; soluble dilute HCl, HBr [HAW93] [MER06] |
| Sensitive | Moisture Sensitive |
| Merck | 14,706 |
| Exposure limits | ACGIH: TWA 0.5 mg/m3 NIOSH: IDLH 50 mg/m3; TWA 0.5 mg/m3 |
| Dielectric constant | 20.899999999999999 |
| CAS DataBase Reference | 7789-61-9(CAS DataBase Reference) |
| EPA Substance Registry System | Antimony tribromide (7789-61-9) |
Safety information for Antimony tribromide
| Pictogram(s) |
 Exclamation Mark Irritant GHS07  Environment GHS09 |
| GHS Hazard Statements |
H302:Acute toxicity,oral H332:Acute toxicity,inhalation H411:Hazardous to the aquatic environment, long-term hazard |
| Precautionary Statement Codes |
P261:Avoid breathing dust/fume/gas/mist/vapours/spray. P264:Wash hands thoroughly after handling. P264:Wash skin thouroughly after handling. P270:Do not eat, drink or smoke when using this product. P304+P340:IF INHALED: Remove victim to fresh air and Keep at rest in a position comfortable for breathing. |
Computed Descriptors for Antimony tribromide
New Products
4-AMINO-TETRAHYDRO-PYRAN-4-CARBOXYLIC ACID HCL 4-(Dimethylamino)tetrahydro-2H-pyran-4-carbonitrile 4-Aminotetrahydropyran-4-carbonitrile Hydrochloride (R)-3-Aminobutanenitrile Hydrochloride 3-((Dimethylamino)methyl)-5-methylhexan-2-one oxalate 1,4-Dioxa-8-azaspiro[4.5]decane 5-Bromo-2-nitropyridine Nimesulide BP Aceclofenac IP/BP/EP Diclofenac Sodium IP/BP/EP/USP Mefenamic Acid IP/BP/EP/USP Ornidazole IP Diclofenac Potassium THOMAIND PAPER PH 2.0 TO 4.5 1 BOX BUFFER CAPSULE PH 9.2 - 10 CAP SODIUM CHLORIDE 0.1N CVS ALLOXAN MONOHYDRATE 98% PLATINUM 0.5% ON 3 MM ALUMINA PELLETS (TYPE 73) LITHIUM AAS SOLUTION 2-Bromo-1-(bromomethyl)-3-chloro-5-nitrobenzene 2-Bromo-3-nitroaniline N-(3-Hydroxypropyl)-N-methylacetamide 3-Bromo-6-chloropyridazine 4-ethyl-3-nitrobenzoic acidRelated products of tetrahydrofuran
You may like
-
 Antimony(III) bromide CAS 7789-61-9View Details
Antimony(III) bromide CAS 7789-61-9View Details
7789-61-9 -
 Antimony(III) bromide CAS 7789-61-9View Details
Antimony(III) bromide CAS 7789-61-9View Details
7789-61-9 -
 Antimony(III) bromide CAS 7789-61-9View Details
Antimony(III) bromide CAS 7789-61-9View Details
7789-61-9 -
 Antimony(III) bromide CAS 7789-61-9View Details
Antimony(III) bromide CAS 7789-61-9View Details
7789-61-9 -
 Antimony(III) bromide CAS 7789-61-9View Details
Antimony(III) bromide CAS 7789-61-9View Details
7789-61-9 -
 Antimony (III) bromide, Ultra dry CAS 7789-61-9View Details
Antimony (III) bromide, Ultra dry CAS 7789-61-9View Details
7789-61-9 -
 Antimony (III) bromide, Ultra dry CAS 7789-61-9View Details
Antimony (III) bromide, Ultra dry CAS 7789-61-9View Details
7789-61-9 -
 Antimony (III) bromide, Ultra dry CAS 7789-61-9View Details
Antimony (III) bromide, Ultra dry CAS 7789-61-9View Details
7789-61-9