Phenanthrene
Synonym(s):[3]Helicene;Phenanthrene;Ravatite
- CAS NO.:85-01-8
- Empirical Formula: C14H10
- Molecular Weight: 178.23
- MDL number: MFCD00001168
- EINECS: 201-581-5
- SAFETY DATA SHEET (SDS)
- Update Date: 2025-09-25 17:15:13
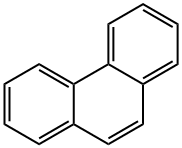
What is Phenanthrene?
Description
Phenanthrene is a polycyclic aromatic hydrocarbon (PAH) composed of three fused benzene rings. The name phenanthrene is a composite of phenyl and anthracene. In its pure form, it is found in cigarette smoke and is a known irritant, photosensitizing skin to light. Phenanthrene appears as a white powder having blue fluorescence.
Chemical properties
Phenanthrene is a white crystalline substance. Weak aromatic odor. Polycyclic aromatic hydrocarbons (PAHs) are compounds containing multiple benzene rings and are also called polynuclear aromatic hydrocarbons.
Physical properties
Colorless, monoclinic crystals with a faint, aromatic odor
The Uses of Phenanthrene
Phenanthrene is a polycyclic aromatic hydrocarbons, an environmental pollutant.
The Uses of Phenanthrene
Labelled polycyclic aromatic hydrocarbons as micropollutants.
The Uses of Phenanthrene
Phenanthrene is a PAH that can be derived from coal tar. Phenanthrene is used in the production of dyes, pharmaceuticals, and explosives, and in biochemical research. A derivative, cyclopentanophenanthrene, has been used as a starting material for synthesizing bile acids, cholesterol, and other steroids.
Definition
ChEBI: A polycyclic aromatic hydrocarbon composed of three fused benzene rings which takes its name from the two terms 'phenyl' and 'anthracene.'
Production Methods
Phenanthrene occurs in coal tar and can be isolated from several types of crude petroleum.
Synthesis Reference(s)
Journal of Heterocyclic Chemistry, 30, p. 291, 1993 DOI: 10.1002/jhet.5570300151
The Journal of Organic Chemistry, 18, p. 801, 1953 DOI: 10.1021/jo50013a004
Tetrahedron Letters, 15, p. 495, 1974
General Description
Colorless monoclinic crystals with a faint aromatic odor. Solutions exhibit a blue fluorescence.
Air & Water Reactions
Insoluble in water.
Reactivity Profile
Phenanthrene may react with oxidizing materials .
Health Hazard
The acute oral toxicity of phenanthrene is low.It is more toxic than anthracene. An oral LD50value in mice is reported at 700 mg/kg. It maycause tumor in skin at the site of application.The evidence of carcinogenicity in animals,however, is inadequate.
Fire Hazard
Phenanthrene is combustible.
Safety Profile
Poison by intravenous route. Moderately toxic by ingestion. Mutation data reported. A human skin photosensitizer. Questionable carcinogen with experimental neoplastigenic and tumorigenic data by skin contact. Combustible when exposed to heat or flame; can react vigorously with oxidizing materials. To fight fire, use water, foam, CO2, dry chemical. When heated to decomposition it emits acrid smoke and irritating fumes
Potential Exposure
Dust may form explosive mixture with air. Incompatible with oxidizers (chlorates, nitrates, peroxides, permanganates, perchlorates, chlorine, bromine, fluorine, etc.); contact may cause fires or explosions. Keep away from alkaline materials, strong bases, strong acids, oxoacids, epoxides.
Carcinogenicity
Phenanthrene is ineffective as an initiator. It is not classifiable as to human carcinogenicity— class 3 by IARC and class D by IRIS, based on no human data and inadequate data from a single gavage study in rats and skin painting and injection studies in mice.
Source
Detected in groundwater beneath a former coal gasification plant in Seattle, WA at a
concentration of 130 μg/L (ASTR, 1995). Detected in 8 diesel fuels at concentrations ranging
from 0.17– 110 mg/L with a mean value of 41.43 mg/L (Westerholm and Li, 1994) and in distilled
water-soluble fractions of new and used motor oil at concentrations of 1.9–2.1 and 2.1–2.2 μg/L,
respectively (Chen et al., 1994). Lee et al. (1992) reported concentration ranges of 100 to 300
mg/L and 15 to 25 μg/L in diesel fuel and the corresponding aqueous phase (distilled water),
respectively. Schauer et al. (1999) reported phenanthrene in diesel fuel at a concentration of 57
μg/g and in a diesel-powered medium-duty truck exhaust at an emission rate of 93.1 μg/km.
Identified in Kuwait and South Louisiana crude oils at concentrations of 26 and 70 ppm,
respectively (Pancirov and Brown, 1975). Diesel fuel obtained from a service station in Schlieren,
Switzerland contained phenanthrene at an estimated concentration of 327 mg/L (Schluep et al.,
2001).
Phenanthrene was detected in asphalt fumes at an average concentration of 57.73 ng/m3 (Wang
et al., 2001).
Thomas and Delfino (1991) equilibrated contaminant-free groundwater collected from
Gainesville, FL with individual fractions of three individual petroleum products at 24–25 °C for
24 h. The aqueous phase was analyzed for organic compounds via U.S. EPA approved test method
625. Phenanthrene was only detected in the water-soluble fraction of diesel fuel at an average
concentration of 17 μg/L.
Based on laboratory analysis of 7 coal tar samples, phenanthrene concentrations ranged from
3,100 to 35,000 ppm (EPRI, 1990). Detected in 1-yr aged coal tar film and bulk coal tar at an
identical concentration of 10,000 mg/kg (Nelson et al., 1996). A high-temperature coal tar
contained phenanthrene at an average concentration of 2.66 wt % (McNeil, 1983). Also identified
in high-temperature coal tar pitches at concentrations ranging from 7,500 to 40,300 mg/kg
(Arrendale and Rogers, 1981). Lee et al. (1992a) equilibrated eight coal tars with distilled water at
25 °C. The maximum concentration of phenanthrene observed in the aqueous phase is 0.4 mg/L.
Nine commercially available creosote samples contained phenanthrene at concentrations
ranging from 48,000 to 120,000 mg/kg (Kohler et al., 2000).
Typical concentration of phenanthrene in a heavy pyrolysis oil is 2.5 wt % (Chevron Phillips,
May 2003).
Environmental Fate
Biological. Catechol is the central metabolite in the bacterial degradation of phenanthrene.
Intermediate by-products include 1-hydroxy-2-naphthoic acid, 1,2-dihydroxynaphthalene, and
salicylic acid (Chapman, 1972; Hou, 1982). It was reported that Beijerinckia, under aerobic
conditions, degraded phenanthrene to cis-3,4-dihydroxy-3,4-dihydrophenanthracene (Kobayashi
and Rittman, 1982).
Soil. The reported half-lives for phenanthrene in a Kidman sandy loam and McLaurin sandy
loam are 16 and 35 d, respectively (Park et al., 1990). Manilal and Alexander (1991) reported a
half-life of 11 d in a Kendaia soil.
Surface Water. In a 5-m deep surface water body, the calculated half-lives for direct photochemical
transformation at 40 °N latitude in the midsummer during midday were 59 and 69 d with
and without sediment-water partitioning, respectively (Zepp and Schlotzhauer, 1979).
Photolytic. A carbon dioxide yield of 24.2% was achieved when phenanthrene adsorbed on
silica gel was irradiated with light (λ >290 nm) for 17 h (Freitag et al., 1985). In a 2-wk
experiment, [14C]phenanthrene applied to soil-water suspensions under aerobic and anaerobic
conditions gave 14CO2 yields of 7.2 and 6.3%, respectively (Scheunert et al., 1987). Matsuzawa et
al. (2001) investigated the photochemical degradation of five polycyclic aromatic hydrocarbons in
diesel particulate matter deposited on the ground and in various soil components. The
photochemical degradation by artificial sunlight was accomplished using a 900-W xenon lamp.
Light from this lamp was passed through a glass filter to eliminate light of shorter wavelengths (λ
<290 nm). The intensity of this light was about 170 mW/cm2. In addition, a solar simulator
equipped with a 300-W xenon lamp was used to provide the maximum sunlight intensity observed
in Tokyo (latitude 35.5 °N). The half-lives of phenanthrene in diesel particulate matter using 900-
and 300-W sources were 4.29 and 60.63 h, respectively. The following half-lives were determined
for phenanthrene adsorbed on various soil components using 900-W apparatus: 3.04 h for quartz,
2.90 h for feldspar, 1.15 h for kaolinite, 4.97 h for montmorillonite, 3.26 h for silica gel, and 1.17
h for alumina.
Chemical/Physical. The aqueous chlorination of phenanthrene at pH <4 produced phenanthrene-
9,10-dione and 9-chlorophenanthrene. At high pH (>8.8), phenanthrene-9,10-oxide, phenanthrene-
9,10-dione, and 9,10-dihydrophenanthrenediol were identified as major products (Oyler et al.,
1983). It was suggested that the chlorination of phenanthrene in tap water accounted for the
presence of chloro- and dichlorophenanthrenes (Shiraishi et al., 1985).
Shipping
UN3077 Environmentally hazardous substances, solid, n.o.s., Hazard class: 9; Labels: 9-Miscellaneous hazardous material, Technical Name Required.
Purification Methods
Likely contaminants include anthracene, carbazole, fluorene and other polycyclic hydrocarbons. Purify it by distillation from sodium under vacuum, boiling with maleic anhydride in xylene, crystallisation from acetic acid, sublimation and zone melting. It has also been recrystallised repeatedly from EtOH, *benzene or pet ether (b 60-70o), with subsequent drying under vacuum over P2O5 in an Abderhalden pistol. Feldman, Pantages and Orchin [J Am Chem Soc 73 4341 1951] separated most of the anthracene impurity by refluxing phenanthrene (671g) with maleic anhydride (194g) in xylene (1.25L) under nitrogen for 22hours, then filtered. The filtrate was extracted with aqueous 10% NaOH, the organic phase was separated, and the solvent was evaporated. The residue, after stirring for 2hours with 7g of sodium, was distilled in a vacuum, then recrystallised twice from 30% *benzene in EtOH. It was then dissolved in hot acetic acid (2.2mL/g), and to it was slowly added an aqueous solution of CrO3 (60g in 72mL H2O plus 2.2L of acetic acid), followed by slow addition of conc H2SO4 (30mL). The mixture was refluxed for 15minutes, diluted with an equal volume of water and cooled. The precipitate was filtered off, washed with water, dried and distilled, then recrystallised twice from EtOH. Further purification is possible by chromatography from a CHCl3 solution on activated alumina, with *benzene as eluent, and by zone refining. The picrate (1:1) forms golden yellow needles with m 146o, and the styphnate (1:1) has m 138-139o (plates or needles from EtOH or EtOH/H2O respectively). [Dornfeld et al. Org Synth Coll Vol III 134 1955, Beilstein 5 H 667, 5 I 327, 5 II 579, 5 III 2136, 5 IV 2297.]
Toxicity evaluation
Phenanthrene absorbs ultraviolet light and causes production of singlet oxygen, which in turn leads to free radical production. Although a large body of literature exists on the toxicity and carcinogenicity of other PAHs, primarily benzo(a)pyrene, toxicity data for phenanthrene are limited.
Waste Disposal
Consult with environmental regulatory agencies for guidance on acceptable disposal practices. Generators of waste containing this contaminant (≥100 kg/mo) must conform with EPA regulations governing storage, transportation, treatment, and waste disposal.
Properties of Phenanthrene
| Melting point: | 98-100 °C (lit.) |
| Boiling point: | 340 °C (lit.) |
| Density | 1.063 g/mL at 25 °C (lit.) |
| vapor density | 6.14 |
| vapor pressure | 0.00012 hPa (20 °C) |
| refractive index | 1.5943 |
| Flash point: | 99-101°C |
| storage temp. | room temp |
| solubility | Soluble in alcohol, benzene, toluene, and glacial acetic acid |
| form | platelets (fine) |
| pka | >15 (Christensen et al., 1975) |
| color | brown |
| Water Solubility | insoluble |
| Merck | 14,7212 |
| BRN | 1905428 |
| Henry's Law Constant | 0.49, 1.80, 3.35, and 7.89 at 5, 15, 25, and 35 °C, respectively (gas stripping-GC, Odabasi et al.,
2006) |
| Exposure limits | OSHA: TWA 0.2 mg/m3 |
| Dielectric constant | 2.72(43.0℃) |
| Stability: | Stable. Combustible. Incompatible with strong oxidizing agents. |
| CAS DataBase Reference | 85-01-8(CAS DataBase Reference) |
| NIST Chemistry Reference | Phenanthrene(85-01-8) |
| IARC | 3 (Vol. Sup 7, 92) 2010 |
| EPA Substance Registry System | Phenanthrene (85-01-8) |
Safety information for Phenanthrene
| Signal word | Warning |
| Pictogram(s) |
 Exclamation Mark Irritant GHS07  Environment GHS09 |
| GHS Hazard Statements |
H302:Acute toxicity,oral H410:Hazardous to the aquatic environment, long-term hazard |
| Precautionary Statement Codes |
P264:Wash hands thoroughly after handling. P264:Wash skin thouroughly after handling. P270:Do not eat, drink or smoke when using this product. P273:Avoid release to the environment. P391:Collect spillage. Hazardous to the aquatic environment P301+P312:IF SWALLOWED: call a POISON CENTER or doctor/physician IF you feel unwell. P501:Dispose of contents/container to..… |
Computed Descriptors for Phenanthrene
Phenanthrene manufacturer
JSK Chemicals
New Products
4,4-Difluoropiperidine hydrochloride tert-butyl 9-methoxy-3-azaspiro[5.5]undecane-3-carboxylate Indole Methyl Resin N-Isopropylurea N,N-Dicyclohexylcarbodiimide(DCC) MELDRUMS ACID 5-METHYLISOXAZOLE-4-CARBOXYLIC ACID Magnessium Bis glycinate Zinc ascorbate 1-bromo-2-butyne 2-acetamidophenol 9(10H)-anthracenone Erythrosin B, 4-Piperidinopiperidine 2-((4-morpholinophenylamino) (methylthio) methylene) malononitrile 2,4-dihydroxybenzaldehyde 3-(4-morpholinophenylamino)-5-amino-1H-pyrazole-4-carbonitrile Methyl 2-methylquinoline-6-carboxylate 2,6-dichloro-4-nitropyridine 4-Bromo-2-chlorobenzonitrile 2-(benzylamino)acetic acid hydrochloride 4-(tert-Butoxycarbonylamino)but- 2-ynoic acid 3,4-dihydro-2H-benzo[b][1,4]dioxepine 1-Phenyl-1-cycloprppanecarboxylicacidRelated products of tetrahydrofuran
You may like
-
 Phenanthrene CAS 85-01-8View Details
Phenanthrene CAS 85-01-8View Details
85-01-8 -
 Phenanthrene, 98%+ CAS 85-01-8View Details
Phenanthrene, 98%+ CAS 85-01-8View Details
85-01-8 -
 Phenanthrene Zone Refined (number of passes:30) CAS 85-01-8View Details
Phenanthrene Zone Refined (number of passes:30) CAS 85-01-8View Details
85-01-8 -
 Phenanthrene CAS 85-01-8View Details
Phenanthrene CAS 85-01-8View Details
85-01-8 -
 Phenanthrene CAS 85-01-8View Details
Phenanthrene CAS 85-01-8View Details
85-01-8 -
 Phenanthrene 98.00% CAS 85-01-8View Details
Phenanthrene 98.00% CAS 85-01-8View Details
85-01-8 -
 Phenanthrene 99% (HPLC) CAS 85-01-8View Details
Phenanthrene 99% (HPLC) CAS 85-01-8View Details
85-01-8 -
 Phenanthrene 98% CAS 85-01-8View Details
Phenanthrene 98% CAS 85-01-8View Details
85-01-8
