Aniline hydrochloride
Synonym(s):Aniline hydrochloride, Aniline hydrochloride;Anilinium chloride
- CAS NO.:142-04-1
- Empirical Formula: C6H8ClN
- Molecular Weight: 129.59
- MDL number: MFCD00012958
- EINECS: 205-519-8
- SAFETY DATA SHEET (SDS)
- Update Date: 2025-08-20 16:56:12
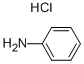
What is Aniline hydrochloride?
Description
Aniline (hydrochloride) (Item No. 21963) is an analytical reference standard that is structurally categorized as a synthetic intermediate. It is used to make the imine derivative of NPP as part of the synthesis of fentanyl (Item Nos. 14719 | ISO60197 ) and its derivatives. This product is intended for research and forensic applications.
Chemical properties
Aniline hydrochloride, a white to greenishcrystalline solid that meltsat 198°C and boils at 245 °C;soluble in water,alcohol, and chloroform; used in making dyes and in printing. Also known as anilinechloride,anilinesalt.
Chemical properties
Aniline is a clear, colorless, oily liquid that darkens on exposure to light; with a characteristic amine-like odor.
The Uses of Aniline hydrochloride
Aniline hydrochloride was used in the preparation of polyaniline coated poly(styrene-co-styrene sulfonate) nanoparticles. It was used to study the induction of Nei-like DNA glycosylases (NEIL1/2)-mediated base excision repair(BER) in rat spleen and 8-oxoguanine glycosylase 1-mediated BER due to aniline exposure. It is used in printing inks, clothing marking inks, paints and paint removers. It is also used as a stove polisher and shoe polisher, as well as in the synthesis of dyes, crayons and antioxidants.
The Uses of Aniline hydrochloride
Intermediates, dyeing and printing, aniline black.
What are the applications of Application
Aniline hydrochloride is The acute toxicity involves its activation in vivo to 4-hydroxyaniline and the formation of adducts with hemoglobin.
General Description
A white to greenish colored crystalline solid. Toxic by ingestion and a skin and eye irritant. May emit toxic aniline and chloride fumes under exposure to high temperatures or flame. Used to make dyes and printing ink.
Air & Water Reactions
Aniline hydrochloride is sensitive to air and light (darkens). Soluble in water and denser than water.
Reactivity Profile
Aniline hydrochloride is incompatible with oxidizing materials.
Health Hazard
TOXIC; inhalation, ingestion or skin contact with material may cause severe injury or death. Contact with molten substance may cause severe burns to skin and eyes. Avoid any skin contact. Effects of contact or inhalation may be delayed. Fire may produce irritating, corrosive and/or toxic gases. Runoff from fire control or dilution water may be corrosive and/or toxic and cause pollution.
Fire Hazard
Combustible material: may burn but does not ignite readily. When heated, vapors may form explosive mixtures with air: indoors, outdoors and sewers explosion hazards. Contact with metals may evolve flammable hydrogen gas. Containers may explode when heated. Runoff may pollute waterways. Substance may be transported in a molten form.
Biochem/physiol Actions
The acute toxicity of aniline involves its activation in vivo to 4-hydroxyaniline and the formation of adducts with hemoglobin. In erythrocytes, this is associated with the release of iron and the accumulation of methemoglobin and the development of hemolytic anemia and inflammation of the spleen. Tumor formation is often observed in the spleen on prolonged administration.
Safety Profile
Suspected carcinogen with experimental carcinogenic and tumorigenic data. Poison by intraperitoneal route. Moderately toxic by ingestion. Experimental teratogenic effects. Human mutation data reported. A skin and eye irritant. Combustible when exposed to heat or flame. When heated to decomposition or on contact with acid or acid fumes, it emits highly toxic fumes of aniline and chlorine compounds. Reacts explosively with aniline at 240℃/7.6 bar. Can react vigorously with oxidizing materials. To fight fire, use water, CO2, water mist or spray, dry chemical. See also ANILINE.
Potential Exposure
Aniline is widely used as an intermediate in the synthesis of dyestuffs. It is also used in the manufacture of rubber accelerators and antioxidants, pharmaceuticals, marking inks; tetryl, optical whitening agents; photographic developers; resins, varnishes, perfumes, shoe polishes, and many organic chemicals.
Shipping
UN1547 Aniline, Hazard Class: 6.1; Labels: 6.1- Poisonous materials. UN1548 Aniline hydrochloride, Hazard Class: 6.1; Labels: 6.1-Poisonous materials.
Purification Methods
Purification is as for aniline HBr above. [Beilstein 12 IV 232.]
Incompatibilities
May form explosive mixture with air. Unless inhibited (usually methanol), aniline is readily able to polymerize. Fires and explosions may result from contact with halogens, strong acids; oxidizers, strong base organic anhydrides; acetic anhydride, isocyanates, aldehydes, sodium peroxide. Strong reaction with toluene diisocyanate. Reacts with alkali metals and alkali earth metals. Attacks some plastics, rubber and coatings; copper and copper alloys.
Waste Disposal
Consult with environmental regulatory agencies for guidance on acceptable disposal practices. Generators of waste containing this contaminant (≥100 kg/mo) must conform with EPA regulations governing storage, transportation, treatment, and waste disposal. Incineration with provision for nitrogen oxides removal from flue gases by scrubber, catalytic or thermal device.
Properties of Aniline hydrochloride
| Melting point: | 196-198 °C(lit.) |
| Boiling point: | 245 °C |
| Density | 1.2215 |
| vapor density | 4.46 (vs air) |
| refractive index | 1.5330 (estimate) |
| Flash point: | 380 °F |
| storage temp. | Store below +30°C. |
| solubility | 1070g/l |
| form | Powder |
| color | White to pale yellow-cream |
| Water Solubility | 1070 g/L (25 ºC) |
| Sensitive | Hygroscopic |
| Merck | 14,659 |
| BRN | 3593823 |
| Stability: | Stable. Incompatible with strong oxidizing agents, strong acids. |
| CAS DataBase Reference | 142-04-1(CAS DataBase Reference) |
| NIST Chemistry Reference | Benzenamine, hydrochloride(142-04-1) |
| IARC | 2A (Vol. 127) |
| EPA Substance Registry System | Aniline hydrochloride (142-04-1) |
Safety information for Aniline hydrochloride
| Signal word | Danger |
| Pictogram(s) |
 Corrosion Corrosives GHS05  Skull and Crossbones Acute Toxicity GHS06  Health Hazard GHS08  Environment GHS09 |
| GHS Hazard Statements |
H317:Sensitisation, Skin H318:Serious eye damage/eye irritation H341:Germ cell mutagenicity H351:Carcinogenicity H372:Specific target organ toxicity, repeated exposure H400:Hazardous to the aquatic environment, acute hazard |
| Precautionary Statement Codes |
P273:Avoid release to the environment. P280:Wear protective gloves/protective clothing/eye protection/face protection. P301+P310:IF SWALLOWED: Immediately call a POISON CENTER or doctor/physician. P305+P351+P338:IF IN EYES: Rinse cautiously with water for several minutes. Remove contact lenses, if present and easy to do. Continuerinsing. |
Computed Descriptors for Aniline hydrochloride
Aniline hydrochloride manufacturer
JSK Chemicals
New Products
Indole Methyl Resin tert-butyl 9-methoxy-3-azaspiro[5.5]undecane-3-carboxylate Boc-His(Boc)-OH 2-CTC Resin 4-Chloro-7-tosy1-7Hpyrrolo[2,3-d]pyrimidine 5,7-Dibromo-1H-indole 2,5-dichloro-N-hydroxy-4,6-dimethylpyridine-3-carboximidamide 2,2-Dimethoxy-7-azaspiro[3.5]nonane hydrochloride 4-chloromethyl-5-methyl-1,3-dioxol-2-one (DMDO-Cl) R-2-BENZYLOXY PROPIONIC ACID 1,1’-CARBONYLDIIMIDAZOLE 1,1’-CARBONYLDI (1,2-4 TRIAZOLE) N-METHYL INDAZOLE-3-CARBOXYLIC ACID 4-((2-hydroxyethyl)thio)benzoic acid 1-(TERT-BUTOXYCARBONYL)-2-PYRROLIDINONE Methyl 6-methylnicotinate 3-Pyridineacrylic acid tert-Butyl carbazate TETRAHYDRO-2H-PYRAN-3-OL 2-((4-morpholinophenylamino) (methylthio) methylene) malononitrile 3-(4-morpholinophenylamino)-5-amino-1H-pyrazole-4-carbonitrile 2,4-dihydroxybenzaldehyde 1,3-Diethyl-1,3-Diphenylurea Methyl 2-methylquinoline-6-carboxylateRelated products of tetrahydrofuran
You may like
-
 Aniline Hydrochloride pure CAS 142-04-1View Details
Aniline Hydrochloride pure CAS 142-04-1View Details
142-04-1 -
 Aniline HCl 98.00% CAS 142-04-1View Details
Aniline HCl 98.00% CAS 142-04-1View Details
142-04-1 -
 Powder Aniline Hydrochloride CAS: 142-04-1View Details
Powder Aniline Hydrochloride CAS: 142-04-1View Details
142-04-1 -
 99% Aniline Hydrochloride PowderView Details
99% Aniline Hydrochloride PowderView Details
142-04-1 -
 Aniline Hydrochloride PowderView Details
Aniline Hydrochloride PowderView Details
142-04-1 -
 ANILINE HYDRO CHLORIDEView Details
ANILINE HYDRO CHLORIDEView Details
142-04-1 -
 Aniline HydrochlorideView Details
Aniline HydrochlorideView Details
142-04-1 -
 ANILINE HYDROCHLORIDE 99% (For Synthesis)View Details
ANILINE HYDROCHLORIDE 99% (For Synthesis)View Details
142-04-1
