Amodiaquine
- CAS NO.:86-42-0
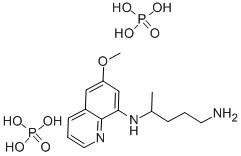
- Empirical Formula: C20H22ClN3O
- Molecular Weight: 355.86
- MDL number: MFCD00552927
- EINECS: 201-669-3
- SAFETY DATA SHEET (SDS)
- Update Date: 2025-12-16 16:15:04
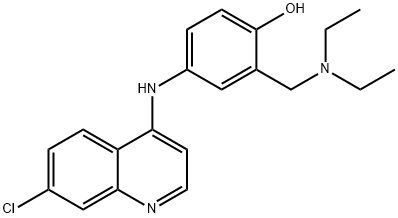
What is Amodiaquine?
Absorption
Rapidly absorbed following oral administration.
Toxicity
LD50 (mouse, intraperitoneal) 225 mg/kg, LD50 (mouse, oral) 550 mg/kg. Symptoms of overdose include headache, drowsiness, visual disturbances, vomiting, hypokalaemia, cardiovascular collapse and cardiac and respiratory arrest. Hypotension, if not treated, may progress rapidly to shock. Electrocardiograms (ECG) may reveal atrial standstill, nodal rhythm, prolonged intraventricular conduction time, broadening of the QRS complex, and progressive bradycardia leading to ventricular fibrillation and/or arrest.
Description
Amodiaquine is a prodrug form of the antimalarial compound N-desethyl amodiaquine . It is active against several strains of P. falciparum in vitro (EC50s = 0.23-0.52 nM) and exhibits a synergistic effect when used in combination with N-desethyl amodiaquine. Amodiaquine dose-dependently inhibits development of parasitemia in a mouse model of P. berghei infection.
Chemical properties
Cyrstalline Solid
Originator
Camoquin HCl,Parke Davis,US,1950
The Uses of Amodiaquine
An antimalarial
Indications
For treatment of acute malarial attacks in non-immune subjects.
What are the applications of Application
Amodiaquine is An anti-malarial and anti-inflammatory compound
Background
A 4-aminoquinoquinoline compound with anti-inflammatory properties.
Definition
ChEBI: A quinoline having a chloro group at the 7-position and an aryl amino group at the 4-position.
Indications
Amodiaquine (Camoquin) is another 4-aminoquinoline derivative whose antimalarial spectrum and adverse reactions are similar to those of chloroquine, although chloroquine-resistant parasites may not be amodiaquine- resistant to the same degree. Prolonged treatment with amodiaquine may result in pigmentation of the palate, nail beds, and skin. There is a 1:2000 risk of agranulocytosis and hepatocellular dysfunction when the drug is used prophylactically.
Manufacturing Process
72.8 g (0.5 mol) of p-aminophenol hydrochloride is dissolved in 500 cc of
water and added to 99 g (0.5 mol) of 4,7-dichloroquinoline. After a few
minutes of warming in a steam bath, 4-(4'-hydroxyanilino)-7-chloroquinoline
hydrochloride, of sufficient purity for use in further experiments, precipitates
as a yellow crystalline solid. Recrystallized from methanol, the MP is over
300°C.
A mixture consisting of 13.5 g of 4-(4'-hydroxyanilino)-7-chloroquinoline
hydrochloride dissolved in absolute ethanol is treated with a solution of 4.38 g of diethylamine and 1.8 g of paraformaldehyde in 20 cc of absolute ethanol.
The reaction mixture is heated under reflux for 16 hours, evaporated to onehalf
volume and the warm solution treated with an excess of hydrogen
chloride dissolved in absolute ethanol. Acetone is added to the warm solution
until it becomes turbid and then the solution is cooled. The crude
dihydrochloride which separates is collected and purified by recrystallization
from methanol; MP 240-242°C.
By using an equivalent amount of 4-(4'-hydroxyanilino)-7-bromoquinoline in
the above procedure, 4-(3'-diethylaminomethyl-4'-hydroxyanilino)-7-
bromoquinoline dihydrochloride is obtained; MP (base) 206-208°C dec.
brand name
Camoquin (Parke-Davis);Amodoquin tablets;Basoquin;Caniquin.
Therapeutic Function
Antimalarial
World Health Organization (WHO)
Amodiaquine, an antimalarial agent related to chloroquine, was introduced over 40 years ago for the treatment and prophylaxis of malaria. The drug was voluntarily withdrawn in the United Kingdom in 1975 for commercial reasons but was subsequently reintroduced in 1985 to meet the medical demand for an antimalarial drug to deal with the rapid spread of chloroquine-resistant falciparum malaria in Asia and Africa. By 1986 a significant number of cases of agranulocytosis associated with prophylactic use, some of which were fatal, had been reported there and it has been estimated that the frequency of this risk is of the order of 1:2,000. Although most cases occurred when amodiaquine had been used in combination with other antimalarials, the major manufacturer decided to withdraw the prophylactic indication worldwide following discussions with experts. Preparations remain available for the treatment of acute attacks of malaria which involves only a short period of exposure to the drug. (Reference: (WHODI) WHO Drug Information, 1, 5, 1987)
Pharmaceutical Applications
A mono-Mannich-base 4-aminoquinoline, formulated as the dihydrochloride dihydrate or free base for oral administration. It is active against P. falciparum and P. vivax and is more active than chloroquine for the treatment of uncomplicated P. falciparum malaria. Chloroquine-resistant strains may remain susceptible, but resistance to amodiaquine is also spreading in some regions of Africa. The pharmacological properties are similar to those of chloroquine. The terminal elimination halflife is 1–3 weeks. It is rapidly and extensively metabolized to the desethyl derivative which has reduced antiplasmodial activity. Prophylactic use has been abandoned because of agranulocytosis and hepatotoxicity due to formation of a quinoneimine metabolite. A fixed dose combination with artesunate and derivatives (for example, isoquine) with altered metabolism and reduced toxicity is in development.
Pharmacokinetics
Amodiaquine, a 4-aminoquinoline similar to chloroquine in structure and activity, has been used as both an antimalarial and an anti-inflammatory agent for more than 40 years. Amodiaquine is at least as effective as chloroquine, and is effective against some chloroquine-resistant strains, although resistance to amodiaquine has been reported. The mode of action of amodiaquine has not yet been determined. 4-Aminoquinolines depress cardiac muscle, impair cardiac conductivity, and produce vasodilatation with resultant hypotension. They depress respiration and cause diplopia, dizziness and nausea.
Clinical Use
Treatment of falciparum malaria
Clinical Use
Mechanistically, it is very similar to chloroquine and does nothave any advantages over the other 4-aminoquinoline drugs.When used for prophylaxis of malaria, it had a higher incidenceof hepatitis and agranulocytosis than that was chloroquine.There is evidence that the hydroquinone (phenol)amine system readily oxidizes to a quinone imine either autoxidatively and/or metabolically, and this productmay contribute to amodiaquine’s toxicity.
Synthesis
Amodiaquin, 4-[(7-chloro-4-quinilyl)amino]-|á-diethylmaino-o-cresol (37.1.1.21), is made by reacting 4,7-dichloroquineoline (37.1.1.1) with 4-aminophenol to make 7-chloro-4-(4-hydroxyphenylamino)-quiniline (37.1.1.20), which then undergoes an aminomethylation reaction using formaldehyde and diethylamine, giving amodiaquin.
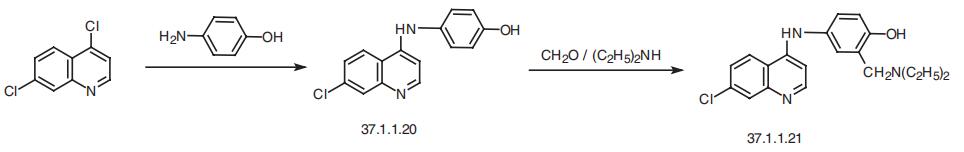
Metabolism
Hepatic biotransformation to desethylamodiaquine (the principal biologically active metabolite) is the predominant route of amodiaquine clearance with such a considerable first pass effect that very little orally administered amodiaquine escapes untransformed into the systemic circulation.
Purification Methods
Amodiaquin crystallises from 2-ethoxyethanol or EtOH. [Burckhalter et al. J Am Chem Soc 70 1363 1948, Beilstein 22 III/IV 4647.]
Properties of Amodiaquine
| Melting point: | 208°C |
| Boiling point: | 478.0±45.0 °C(Predicted) |
| Density | 1.258 |
| storage temp. | -20°C Freezer |
| solubility | DMSO (Slightly, Sonicated), Methanol (Slightly) |
| form | Solid |
| pka | 9.43±0.50(Predicted) |
| color | Pale Yellow to Light Yellow |
| CAS DataBase Reference | 86-42-0(CAS DataBase Reference) |
| NIST Chemistry Reference | Amodiaquine(86-42-0) |
Safety information for Amodiaquine
Computed Descriptors for Amodiaquine
Amodiaquine manufacturer
New Products
Indole Methyl Resin tert-butyl 9-methoxy-3-azaspiro[5.5]undecane-3-carboxylate Boc-His(Boc)-OH 2-CTC Resin 4-Chloro-7-tosy1-7Hpyrrolo[2,3-d]pyrimidine 5,7-Dibromo-1H-indole 2,5-dichloro-N-hydroxy-4,6-dimethylpyridine-3-carboximidamide 2,2-Dimethoxy-7-azaspiro[3.5]nonane hydrochloride 4-chloromethyl-5-methyl-1,3-dioxol-2-one (DMDO-Cl) R-2-BENZYLOXY PROPIONIC ACID 1,1’-CARBONYLDIIMIDAZOLE 1,1’-CARBONYLDI (1,2-4 TRIAZOLE) N-METHYL INDAZOLE-3-CARBOXYLIC ACID 4-((2-hydroxyethyl)thio)benzoic acid 1-(TERT-BUTOXYCARBONYL)-2-PYRROLIDINONE Methyl 6-methylnicotinate 3-Pyridineacrylic acid tert-Butyl carbazate TETRAHYDRO-2H-PYRAN-3-OL 2-((4-morpholinophenylamino) (methylthio) methylene) malononitrile 3-(4-morpholinophenylamino)-5-amino-1H-pyrazole-4-carbonitrile 2,4-dihydroxybenzaldehyde 1,3-Diethyl-1,3-Diphenylurea Methyl 2-methylquinoline-6-carboxylateRelated products of tetrahydrofuran





You may like
-
 86-42-0 99%View Details
86-42-0 99%View Details
86-42-0 -
 Amodiaquine 98%View Details
Amodiaquine 98%View Details
86-42-0 -
 86-42-0 Amodiaquine 98%View Details
86-42-0 Amodiaquine 98%View Details
86-42-0 -
 86-42-0 Amodiaquine 98%View Details
86-42-0 Amodiaquine 98%View Details
86-42-0 -
 Amodiaquine 98.00% CAS 86-42-0View Details
Amodiaquine 98.00% CAS 86-42-0View Details
86-42-0 -
 Amodiaquine APIView Details
Amodiaquine APIView Details
86-42-0 -
 Amodiaquine, Packaging Size: 25kg, Packaging Type: Hdpe DrumView Details
Amodiaquine, Packaging Size: 25kg, Packaging Type: Hdpe DrumView Details
6398-98-7 -
 AmodiaquineView Details
AmodiaquineView Details
86-42-0
