CHEMICAL AND PHYSICAL PROPERTIES
| Physical Description | Solid |
|---|---|
| Color/Form | Crystals from absolute ethanol |
| Melting Point | 206-208 |
| Solubility | 24.9 [ug/mL] (The mean of the results at pH 7.4) |
| LogP | 3.7 |
| Collision Cross Section | 191.9 Ų [M+H]+ [CCS Type: TW, Method: calibrated with polyalanine and drug standards] |
| Other Experimental Properties | Yellow bitter crystals. Decomposes at 150-160 °C. UV max (methanol) 342 nm; 341.5 nm (water). Soluble in water, sparingly soluble in alcohol. Very slightly soluble in benzene, chloroform, and ether. pH 1% aqueous soln = 4-4.8. /Amodiaquine dihydrochloride dihydrate/ |
COMPUTED DESCRIPTORS
| Molecular Weight | 355.9 g/mol |
|---|---|
| XLogP3 | 2.6 |
| Hydrogen Bond Donor Count | 2 |
| Hydrogen Bond Acceptor Count | 4 |
| Rotatable Bond Count | 6 |
| Exact Mass | 355.1451400 g/mol |
| Monoisotopic Mass | 355.1451400 g/mol |
| Topological Polar Surface Area | 48.4 Ų |
| Heavy Atom Count | 25 |
| Formal Charge | 0 |
| Complexity | 406 |
| Isotope Atom Count | 0 |
| Defined Atom Stereocenter Count | 0 |
| Undefined Atom Stereocenter Count | 0 |
| Defined Bond Stereocenter Count | 0 |
| Undefined Bond Stereocenter Count | 0 |
| Covalently-Bonded Unit Count | 1 |
| Compound Is Canonicalized | Yes |
PRODUCT INTRODUCTION
description
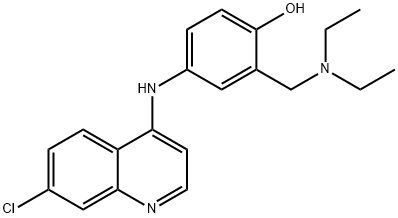
Amodiaquine is a quinoline having a chloro group at the 7-position and an aryl amino group at the 4-position. It has a role as an antimalarial, a non-steroidal anti-inflammatory drug, a drug allergen, a prodrug, an EC 2.1.1.8 (histamine N-methyltransferase) inhibitor and an anticoronaviral agent. It is a member of phenols, an aminoquinoline, a secondary amino compound, a tertiary amino compound and an organochlorine compound. It is a conjugate base of an amodiaquine(1+).