Ammonium citrate dibasic
Synonym(s):di-Ammonium hydrogen citrate;Diammonium hydrogen citrate;Citric acid ammonium salt;Citric acid triammonium salt;Ammonium citrate dibasic, Diammonium hydrogen citrate
- CAS NO.:3012-65-5
- Empirical Formula: C6H14N2O7
- Molecular Weight: 226.18
- MDL number: MFCD00013068
- EINECS: 221-146-3
- SAFETY DATA SHEET (SDS)
- Update Date: 2025-12-17 09:49:14
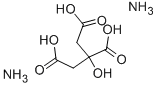
What is Ammonium citrate dibasic?
Chemical properties
white crystalline powder
Chemical properties
Ammonium citrate (various forms) is a white powdery material with a slight ammonia-like odor.
The Uses of Ammonium citrate dibasic
Ammonium citrate dibasic is generally used as a component of matrix solution in matrix-assisted laser desorption/ionization time-of-flight (MALDI-TOF) mass spectrometry analysis. It can also be used as both the carbon and nitrogen source to synthesize fluorescent nitrogen-doped carbon nanoparticles (CNPs).
The Uses of Ammonium citrate dibasic
Determination of phosphates.
The Uses of Ammonium citrate dibasic
For the determination of phosphate, especially in fertilizers.
The Uses of Ammonium citrate dibasic
Ammonium Citrate, Dibasic is a flavor enhancer and ph control agent. it is the diammonium salt of citric acid, being prepared by partially neutralizing citric acid with ammonia. it is used in nonal- coholic beverages and certain cheeses.
What are the applications of Application
Ammonium hydrogencitrate is an electrolyte support compound for MALDI matricies
Definition
ChEBI: A citrate salt in which two of the three carboxy groups are deprotonated and associated with ammonium ions as counter-cations.
General Description
Ammonium citrate is a white granular solid. Ammonium citrate dibasic is soluble in water. The primary hazard is the threat to the environment. Immediate steps should be taken to limit its spread to the environment. Ammonium citrate dibasic is used in pharmaceuticals, and in chemical analysis.
Air & Water Reactions
Water soluble.
Reactivity Profile
Salts, basic, such as AMMONIUM CITRATE, are generally soluble in water. The resulting solutions contain moderate concentrations of hydroxide ions and have pH's greater than 7.0. They react as bases to neutralize acids. These neutralizations generate heat, but less or far less than is generated by neutralization of the bases in reactivity group 10 (Bases) and the neutralization of amines. They usually do not react as either oxidizing agents or reducing agents but such behavior is not impossible.
Health Hazard
Inhalation causes respiratory irritation. Ingestion causes diarrhea. Contact with eyes causes mild irritation.
Fire Hazard
Special Hazards of Combustion Products: Toxic ammonia gas may form in fire.
Potential Exposure
It is used to make pharmaceuticals; rust-proofing compounds and plasticizers; as a food additive; stabilizing agent.
Shipping
UN3077 Environmentally hazardous substances, solid, n.o.s., Hazard class: 9; Labels: 9-Miscellaneous hazardous material, Technical Name Required.
Incompatibilities
Contact with strong caustics causes the release of ammonia gas.
Waste Disposal
May be flushed to sewer with large volumes of water.
Properties of Ammonium citrate dibasic
| Melting point: | 185 °C (dec.)(lit.) |
| Boiling point: | 100 °C(lit.) |
| Density | 1.22 g/mL at 20 °C |
| vapor density | 1.8 (vs air) |
| refractive index | 1.4650 (estimate) |
| storage temp. | Store at +5°C to +30°C. |
| solubility | H2O: 1 M at 20 °C, clear, colorless |
| form | Solid |
| color | White |
| PH | 5.48(1 mM solution);5.29(10 mM solution);5.04(100 mM solution);6.16(1000 mM solution) |
| Odor | Slight ammonia odor |
| PH Range | 5.2 |
| Water Solubility | Soluble in water. Slightiy soluble in alcohol. |
| λmax | λ: 260 nm Amax: ≤0.05 λ: 280 nm Amax: ≤0.03 |
| Merck | 14,512 |
| BRN | 4925760 |
| Stability: | Stable. |
| CAS DataBase Reference | 3012-65-5(CAS DataBase Reference) |
| EPA Substance Registry System | Diammonium citrate (3012-65-5) |
Safety information for Ammonium citrate dibasic
| Signal word | Warning |
| Pictogram(s) |
 Exclamation Mark Irritant GHS07 |
| GHS Hazard Statements |
H315:Skin corrosion/irritation H319:Serious eye damage/eye irritation |
| Precautionary Statement Codes |
P264:Wash hands thoroughly after handling. P264:Wash skin thouroughly after handling. P280:Wear protective gloves/protective clothing/eye protection/face protection. P302+P352:IF ON SKIN: wash with plenty of soap and water. P305+P351+P338:IF IN EYES: Rinse cautiously with water for several minutes. Remove contact lenses, if present and easy to do. Continuerinsing. P332+P313:IF SKIN irritation occurs: Get medical advice/attention. P337+P313:IF eye irritation persists: Get medical advice/attention. |
Computed Descriptors for Ammonium citrate dibasic
| InChIKey | YXVFQADLFFNVDS-UHFFFAOYSA-N |
Ammonium citrate dibasic manufacturer
New Products
4,4-Difluoropiperidine hydrochloride tert-butyl 9-methoxy-3-azaspiro[5.5]undecane-3-carboxylate Indole Methyl Resin N-Isopropylurea N,N-Dicyclohexylcarbodiimide(DCC) MELDRUMS ACID 5-METHYLISOXAZOLE-4-CARBOXYLIC ACID Magnessium Bis glycinate Zinc ascorbate 1-bromo-2-butyne 2-acetamidophenol 9(10H)-anthracenone Erythrosin B, 4-Piperidinopiperidine 2-((4-morpholinophenylamino) (methylthio) methylene) malononitrile 2,4-dihydroxybenzaldehyde 3-(4-morpholinophenylamino)-5-amino-1H-pyrazole-4-carbonitrile Methyl 2-methylquinoline-6-carboxylate 2,6-dichloro-4-nitropyridine 4-Bromo-2-chlorobenzonitrile 2-(benzylamino)acetic acid hydrochloride 4-(tert-Butoxycarbonylamino)but- 2-ynoic acid 3,4-dihydro-2H-benzo[b][1,4]dioxepine 1-Phenyl-1-cycloprppanecarboxylicacidRelated products of tetrahydrofuran


You may like
-
 Ammonium citrate, dibasic 98%View Details
Ammonium citrate, dibasic 98%View Details -
 Ammonium citrate, tribasic 98%View Details
Ammonium citrate, tribasic 98%View Details -
 Citric acid diammonium salt CAS 3012-65-5View Details
Citric acid diammonium salt CAS 3012-65-5View Details
3012-65-5 -
 Citric acid diammonium salt CAS 3012-65-5View Details
Citric acid diammonium salt CAS 3012-65-5View Details
3012-65-5 -
 Citric acid diammonium salt CAS 3012-65-5View Details
Citric acid diammonium salt CAS 3012-65-5View Details
3012-65-5 -
 Citric acid diammonium salt CAS 3012-65-5View Details
Citric acid diammonium salt CAS 3012-65-5View Details
3012-65-5 -
 Citric acid diammonium salt CAS 3012-65-5View Details
Citric acid diammonium salt CAS 3012-65-5View Details
3012-65-5 -
 Citric acid diammonium salt CAS 3012-65-5View Details
Citric acid diammonium salt CAS 3012-65-5View Details
3012-65-5
