631-61-8
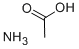
Product Name:
Ammonium acetate
Formula:
C2H7NO2
Synonyms:
Acetic Acid, Ammonium Salt;Ammonium acetate;Ammonium ethanoate;buffers
Inquiry
CHEMICAL AND PHYSICAL PROPERTIES
| Physical Description | Ammonium acetate appears as a white crystalline solid. The primary hazard is the threat to the environment. Immediate steps should be taken to limit its spread to the environment. It is used in chemical analysis, in pharmaceuticals, in preserving foods, and for other uses. |
|---|---|
| Color/Form | CRYSTALS OR CRYSTALLINE MASSES |
| Odor | SLIGHT ACETOUS ODOR |
| Melting Point | 237.2 °F (USCG, 1999) |
| Solubility | SOL IN LESS THAN 1 PART WATER; FREELY SOL IN ALCOHOL; SLIGHTLY SOL IN ACETONE. |
| Density | 1.17 at 68 °F (USCG, 1999) - Denser than water; will sink |
| Vapor Pressure | 0.00014 [mmHg] |
| Stability/Shelf Life | DELIQUESCENT; TENDS TO LOSE AMMONIA. |
| pH | VERY CONCENTRATED SOLN IS SLIGHTLY ACID & 0.5 MOLAR AQ SOLN HAS PH 7.0. |
| Chemical Classes | Nitrogen Compounds -> Ammonium Compounds |
SAFETY INFORMATION
| Signal word | Warning |
|---|---|
| Pictogram(s) |
 Exclamation Mark Irritant GHS07 |
| GHS Hazard Statements |
H319:Serious eye damage/eye irritation |
| Precautionary Statement Codes |
P305+P351+P338:IF IN EYES: Rinse cautiously with water for several minutes. Remove contact lenses, if present and easy to do. Continuerinsing. |
COMPUTED DESCRIPTORS
| Molecular Weight | 77.08 g/mol |
|---|---|
| Hydrogen Bond Donor Count | 1 |
| Hydrogen Bond Acceptor Count | 2 |
| Rotatable Bond Count | 0 |
| Exact Mass | 77.047678466 g/mol |
| Monoisotopic Mass | 77.047678466 g/mol |
| Topological Polar Surface Area | 41.1 Ų |
| Heavy Atom Count | 5 |
| Formal Charge | 0 |
| Complexity | 25.5 |
| Isotope Atom Count | 0 |
| Defined Atom Stereocenter Count | 0 |
| Undefined Atom Stereocenter Count | 0 |
| Defined Bond Stereocenter Count | 0 |
| Undefined Bond Stereocenter Count | 0 |
| Covalently-Bonded Unit Count | 2 |
| Compound Is Canonicalized | Yes |
PRODUCT INTRODUCTION
description
Ammonium acetate is a white crystalline (gritty) solid with a slight vinegar flavour. It functions as a food acidity regulator and buffer. It is used in chemical analysis, textile dyeing and meat preservation. It is also used in pharmaceuticals and drug manufacturing as a diuretic and antipyretic.
