Aluminium hydride
- CAS NO.:7784-21-6
- Empirical Formula: AlH3
- Molecular Weight: 30.005358
- MDL number: MFCD00003417
- EINECS: 232-053-2
- SAFETY DATA SHEET (SDS)
- Update Date: 2024-12-18 14:08:52

What is Aluminium hydride?
Chemical properties
colorless, nonvolatile solid; can be obtained by reacting an ether solution of AlCl3 with LiH; used as a catalyst for organic polymerization processes [MER06]
Physical properties
Colorless cubic crystal; very unstable; decomposes in water; ?Η°? ?11.0 kcal/mol (-46.0kJ/mol).
The Uses of Aluminium hydride
As catalyst for polymerizations; reducing agent. Lithium aluminum hydride, q.v. is a more powerful reagent because of its greater soly.
The Uses of Aluminium hydride
Aluminum Hydride is a relatively unstable polymeric covalent hydride that received considerable attention in the mid- 1960s because of its potential as a high energy additive to solid rocket propellants. The projected uses, including aluminum plating, never materialized, and in spite of intense research and development, commercial manufacture has not been undertaken. The synthetic methods developed were costly.
Preparation
Aluminum hydride is prepared by the reaction of lithium hydride with aluminum chloride in diethyl ether.
3LiH + AlCl3 → AlH3 + 3LiCl
Definition
ChEBI: Alumane is an aluminium hydride and a mononuclear parent hydride.
General Description
A colorless to white solid.
Air & Water Reactions
Ignites in moist air. Ignites in air with or without oxygen enrichment [Bretherick 1979 p. 221]. Explosively hydrolyzed by water (forms hydrogen gas) [Ruff J.K. Inorg. Synth 1967, 9, 34].
Reactivity Profile
Aluminium hydride is a powerful reducing agent. May react violently with oxidizers. Prolonged exposure to heat may cause spontaneous decomposition. Can also decompose spontaneously at ambient temperature with explosive violence. Occasionally, explosions have occurred when Aluminium hydride was stored in ether. The explosions have been blamed on the presence of carbon dioxide impurity in the ether [J. Amer. Chem. Soc. 70:877 1948]. Can emit toxic fumes on contact with acid or fumes from an acid. [Lewis]. At elevated temperatures, the hydride reduces carbon dioxide or sodium hydrogen carbonate to methane and ethane. These gases are the explosive products formed when CO2 extinguishers have been used during hydride fires. The 1:1 complexes of the hydride (as a complex with ether or dimethylamine) and various tetrazole derivatives are explosive. Tetrazoles include, 2-methyl, 2-ethyl, 5-ethyl, 2-methyl-5-vinyl, 5-amino-2-ethyl, etc., [US Pat. 3 396 170, 1968].
Health Hazard
Inhalation or contact with vapors, substance or decomposition products may cause severe injury or death. May produce corrosive solutions on contact with water. Fire will produce irritating, corrosive and/or toxic gases. Runoff from fire control may cause pollution.
Fire Hazard
Produce flammable gases on contact with water. May ignite on contact with water or moist air. Some react vigorously or explosively on contact with water. May be ignited by heat, sparks or flames. May re-ignite after fire is extinguished. Some are transported in highly flammable liquids. Runoff may create fire or explosion hazard.
Safety Profile
Hydrides of some metals (such as ASH3 are extremely toxic. Dangerous fire hazard. An unstable material which is spontaneously flammable in air or O2. Evolves explosive H2 upon contact with moisture. Severe explosion hazard by chemical reaction wherein H2 gas is produced, also in contact with methyl ethers contaminated by Con. Mixtures with tetrazole derivatives are explosive. Reacts with oxidzing materials. On contact with acid or acid fumes, it can emit toxic fumes. See also HYDRIDES and ALUMINUM COMPOUNDS.
Properties of Aluminium hydride
| Melting point: | decomposes at 160℃ [HAW93] |
| Density | 1.45 |
| solubility | reacts with H2O |
| form | colorless hexagonal crystals |
| color | colorless hexagonal, hexane crystals, crystalline |
| Water Solubility | evolves H2 in H2O [HAW93] |
| CAS DataBase Reference | 7784-21-6 |
| EPA Substance Registry System | Aluminum trihydride (7784-21-6) |
Safety information for Aluminium hydride
Computed Descriptors for Aluminium hydride
New Products
4,4-Difluoropiperidine hydrochloride tert-butyl 9-methoxy-3-azaspiro[5.5]undecane-3-carboxylate Indole Methyl Resin N-Isopropylurea N,N-Dicyclohexylcarbodiimide(DCC) MELDRUMS ACID 5-METHYLISOXAZOLE-4-CARBOXYLIC ACID Magnessium Bis glycinate Zinc ascorbate 1-bromo-2-butyne 2-acetamidophenol 9(10H)-anthracenone Erythrosin B, 4-Piperidinopiperidine 2-((4-morpholinophenylamino) (methylthio) methylene) malononitrile 2,4-dihydroxybenzaldehyde 3-(4-morpholinophenylamino)-5-amino-1H-pyrazole-4-carbonitrile Methyl 2-methylquinoline-6-carboxylate 2,6-dichloro-4-nitropyridine 4-Bromo-2-chlorobenzonitrile 2-(benzylamino)acetic acid hydrochloride 4-(tert-Butoxycarbonylamino)but- 2-ynoic acid 3,4-dihydro-2H-benzo[b][1,4]dioxepine 1-Phenyl-1-cycloprppanecarboxylicacidRelated products of tetrahydrofuran
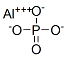
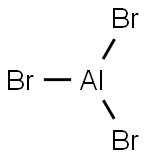
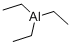
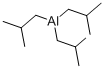

You may like
-
 3-(4-amino-1-oxoisoindolin-2-yl)-1-methylpiperidine-2,6-dione 98%View Details
3-(4-amino-1-oxoisoindolin-2-yl)-1-methylpiperidine-2,6-dione 98%View Details -
 1-methylindoline-2,3-dione 98%View Details
1-methylindoline-2,3-dione 98%View Details
2058-74-4 -
 614-19-7 98%View Details
614-19-7 98%View Details
614-19-7 -
 3112-85-4 Methyl phenyl sulfone 98%View Details
3112-85-4 Methyl phenyl sulfone 98%View Details
3112-85-4 -
 20677-73-0 (2,2-diethoxyethyl)methylamine 98%View Details
20677-73-0 (2,2-diethoxyethyl)methylamine 98%View Details
20677-73-0 -
 3-(4-(hydroxyamino)-1-oxoisoindolin-2-yl)piperidine-2,6-dione 98%View Details
3-(4-(hydroxyamino)-1-oxoisoindolin-2-yl)piperidine-2,6-dione 98%View Details -
 57381-49-4 2-bromo-4-chlorobenzonitrile 98%View Details
57381-49-4 2-bromo-4-chlorobenzonitrile 98%View Details
57381-49-4 -
 4,6-dichloropyrimidine-5-carbaldehyde 98%View Details
4,6-dichloropyrimidine-5-carbaldehyde 98%View Details
5305-40-8
