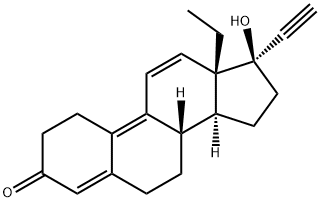
Altrenogest
Synonym(s):17-α-Allyl-17-β-hydroxyoestra-4,9,11-trien-3-one;Allyltrenbolone;Regumate
- CAS NO.:850-52-2
- Empirical Formula: C21H26O2
- Molecular Weight: 310.44
- MDL number: MFCD00867855
- EINECS: 212-703-1
- SAFETY DATA SHEET (SDS)
- Update Date: 2025-04-16 17:28:31
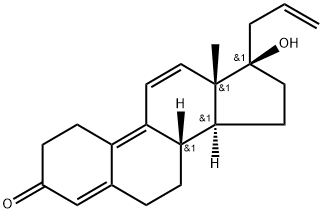
What is Altrenogest?
Chemical properties
Pale Yellow Solid
The Uses of Altrenogest
Altrenogest can be used as a synthetic progestational agent used in veterinary medicine for the control of estrus in mares and antineoplastic.
What are the applications of Application
Altrenogest is a synthetic trienic C21 steroidal progestomimetic
Definition
ChEBI: Altrenogest is a 3-hydroxy steroid.
brand name
REGUMATE (Roussel-UCLAF, France).
Side Effects
People exposed to altrenogest products have potential health risks. Reproductive adverse effects reported in women and girls include abnormal or absent menstrual cycles, and in men include decreased libido. Other adverse effects reported after exposure include headaches, fever, abdominal pain, nausea, diarrhea, vomiting, and rashes.
Altrenogest causes essentially no side effects in mares, and it may be used for prolonged periods.
Veterinary Drugs and Treatments
Altrenogest (Regu-Mate) is indicated (labeled) to suppress estrus
in mares to allow a more predictable occurrence of estrus following
withdrawal of the drug. It is used clinically to assist mares to establish
normal cycles during the transitional period from anestrus
to the normal breeding season often in conjunction with an artificial
photoperiod. It is more effective in assisting in pregnancy attainment
later in the transition period. Some authors (Squires et
al. 1983) suggest selecting mares with considerable follicular activity
(mares with one or more follicles 20 mm or greater in size) for
treatment during the transitional phase. Mares that have been in
estrus for 10 days or more and have active ovaries are also considered
excellent candidates for progestin treatment.
Altrenogest is effective in normally cycling mares for minimizing
the necessity for estrus detection, for the synchronization of estrus,
and permitting scheduled breeding. Estrus will ensue 2 – 5 days after
treatment is completed and most mares ovulate between 8 – 15
days after withdrawal. Altrenogest is also effective in suppressing estrus expression in show mares or mares to be raced. Although the
drug is labeled as contraindicated during pregnancy, it has been
demonstrated to maintain pregnancy in oophorectomized mares
and may be of benefit in mares that abort due to sub-therapeutic
progestin
levels.
The product Matrix is labeled for synchronization of estrus
in sexually mature gilts that have had at least one estrous cycle.
Treatment with altrenogest results in estrus (standing heat) 4-9 days
after completion of the 14-day treatment period.
Altrenogest has been used in dogs for luteal insufficiency and as a
treatment to prevent premature delivery.
Properties of Altrenogest
| Melting point: | 120° |
| Boiling point: | 390.58°C (rough estimate) |
| alpha | D20 -72° (c = 0.5 in ethanol) |
| Density | 1.0865 (rough estimate) |
| refractive index | 1.4900 (estimate) |
| storage temp. | Sealed in dry,2-8°C |
| solubility | DMF: 2 mg/ml; DMSO: 0.1 mg/ml; Ethanol: 20 mg/ml; Ethanol:PBS (pH 7.2)(1:2): 0.3 mg/ml |
| pka | 14.59±0.40(Predicted) |
| form | Solid |
| form | neat |
| color | White to Yellow |
| Merck | 14,319 |
| InChI | InChI=1/C21H26O2/c1-3-10-21(23)12-9-19-18-6-4-14-13-15(22)5-7-16(14)17(18)8-11-20(19,21)2/h3,8,11,13,18-19,23H,1,4-7,9-10,12H2,2H3/t18-,19+,20+,21+/s3 |
Safety information for Altrenogest
| Signal word | Danger |
| Pictogram(s) |
 Skull and Crossbones Acute Toxicity GHS06  Health Hazard GHS08  Environment GHS09 |
| GHS Hazard Statements |
H301:Acute toxicity,oral H373:Specific target organ toxicity, repeated exposure H410:Hazardous to the aquatic environment, long-term hazard |
| Precautionary Statement Codes |
P202:Do not handle until all safety precautions have been read and understood. P260:Do not breathe dust/fume/gas/mist/vapours/spray. P264:Wash hands thoroughly after handling. P264:Wash skin thouroughly after handling. P270:Do not eat, drink or smoke when using this product. P273:Avoid release to the environment. P301+P310:IF SWALLOWED: Immediately call a POISON CENTER or doctor/physician. |
Computed Descriptors for Altrenogest
| InChIKey | VWAUPFMBXBWEQY-ANULTFPQSA-N |
| SMILES | C[C@]12C=CC3=C4CCC(=O)C=C4CC[C@@]3([H])[C@]1([H])CC[C@@]2(O)CC=C |&1:1,14,16,20,r| |
Altrenogest manufacturer
New Products
8-Bromoisoquinoline 3-pyridine carboxaldehyde 7-bromoquinoxalin-2(1H)-one 1-Benzyl-4-[(5,6-dimethoxy-1-oxoindan-2-ylidene)methyl]piperidine 3-Pyridineacetonitrile, α-amino-, hydrochloride (1:1) Trans-methyl 4-aminocyclohexane- carboxylate HCl 4-[2-(DIMETHYLAMINO)ETHOXY]BENZYLAMINE Xanomeline Suzetrigine 5-(5,5-Dimethyl-1,3,2-dioxaborinan-2-yl)-3-methyl-1,3-benzoxazol-2(3H)-one Dimethyl 4-aminothiophene-2,3-dicarboxylate hydrochloride Remibrutinib 1H-Indole-1-carboxylic acid, 5-hydroxy-7-methyl-, 1,1-dimethylethyl ester N N N'Trimethyl ethylenediamine Variamine Blue B Diazonium salt Lead II Bromide N N' DimethylEthylenediamine Ethyl Methanesulfonate N Ethylmethylamine Zinc Chloride Solution (All Grades) Calcium Alphaketoglutarate* L-Glycine methyl ester.HCl H-Ser(t-Bu)-Ser(t-Bu)-Gly-OH Fmoc-L-Glu(OtBu)-OH.H2ORelated products of tetrahydrofuran

You may like
-
 Altrenogest CAS 850-52-2View Details
Altrenogest CAS 850-52-2View Details
850-52-2 -
 Altrenogest CAS 850-52-2View Details
Altrenogest CAS 850-52-2View Details
850-52-2 -
 2-Fluoro-6-iodobenzoic acid 111771-08-5 98+View Details
2-Fluoro-6-iodobenzoic acid 111771-08-5 98+View Details
111771-08-5 -
![3-(4-bromo-3-methyl-2-oxo-2,3-dihydro-1H-benzo[d]imidazol-1-yl)piperidine-2,6-dione 98+](https://img.chemicalbook.in//Content/image/CP5.jpg) 3-(4-bromo-3-methyl-2-oxo-2,3-dihydro-1H-benzo[d]imidazol-1-yl)piperidine-2,6-dione 98+View Details
3-(4-bromo-3-methyl-2-oxo-2,3-dihydro-1H-benzo[d]imidazol-1-yl)piperidine-2,6-dione 98+View Details
2304754-51-4 -
 2,3-Difluoro-6-methoxybenzyl Chloride 1073435-67-2 98+View Details
2,3-Difluoro-6-methoxybenzyl Chloride 1073435-67-2 98+View Details
1073435-67-2 -
 2-(6-(benzyloxy)-3,4-dihydronaphthalen-2-yl)-4,4,5,5-tetramethyl-1,3,2-dioxaborolane 98+View Details
2-(6-(benzyloxy)-3,4-dihydronaphthalen-2-yl)-4,4,5,5-tetramethyl-1,3,2-dioxaborolane 98+View Details
2477812-43-2 -
 KT-474 98+View Details
KT-474 98+View Details
2432994-31-3 -
 7646-85-7 Zinc Chloride Solution (All Grades) As requiredView Details
7646-85-7 Zinc Chloride Solution (All Grades) As requiredView Details
7646-85-7
