Allylestrenol
- CAS NO.:432-60-0
- Empirical Formula: C21H32O
- Molecular Weight: 300.48
- MDL number: MFCD00198957
- EINECS: 207-082-9
- SAFETY DATA SHEET (SDS)
- Update Date: 2025-12-29 14:06:09
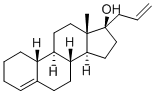
What is Allylestrenol?
Originator
Alilestrenol ,Terapia
The Uses of Allylestrenol
Allylestrenol, is a synthetic sexualsteroid, that is used worldwide in case of endangered pregnancies.
The Uses of Allylestrenol
A synthetic steroid with progestational activity.
Definition
ChEBI: Allylestrenol is a steroid. It derives from a hydride of an estrane.
Manufacturing Process
To 145 ml of dry methylamine which is cooled to -20°C 1.5 g of lithium cut to small pieces are added. To the solution which is blue in color after 10-20 min,
a solution of 3.0 g of oestradiol-3-methylether in 145 ml of absolute ether is
added drop wise. Subsequently the reaction mixture is stirred at -10°C for 40
h, after which 50 ml of absolute ethanol are added. Then the methylamine is
distilled off at law pressure.
To the remaining solution 50 ml of ether and 50 ml of water are added. The
water layer is separated and extracted with ether. The ethereal layer is
washed with a 2 N hydrochloric acid solution, subsequently with a saturated
sodium bicarbonate solution, and then with water. The ethereal solution is
dried and evaporated to dryness. The resulting crude reaction product is
dissolved in a mixture of benzene and petroleum ether (1:3) and
chromatographed over aluminium oxide. The δ4-17β-hydroxy-oestrene
obtained after chromatographic purification has a melting point of 80°-90°C
and 95°-100°C after repeated crystallization from petroleum ether.
A solution of 13.2 g of chromium trioxide in a mixture of 120 ml of water and
20 ml of acetic acid is added, with stirring, to a solution of 20 g of δ4-17β-
hydroxy-oestrene in 400 ml of benzene. Subsequently the reaction mixture is
vigorously stirred at room temperature for 16 h, after which the benzene layer
is separated.
The remaining aqueous layer is extracted a few times with benzene and the
benzene extracts collected are then added to the separated benzene layer.
The benzene extracts are successively washed with dilute sulfuric acid and
water and then evaporated to dryness. The residue is crystallized from
acetone, and the δ4-17β-oxo-oestrene, melting point 114°-116°C is obtained.
To a mixture of 22.4 ml of absolute ether and 1.84 g of magnesium, a mixture
of 2.72 ml of allyl bromide and 2.72 ml of absolute ether is added in nitrogen
atmosphere. Subsequently a solution of 2 g of δ4-17β-oxo-oestrene in 30 ml
of absolute ether is added to this reaction mixture, after which the whole is
stirred for 4 h. Then the reaction mixture is poured into acidified ice water.
The aqueous mixture is extracted with ether; the ether layer is separated,
washed with water, dried over sodium sulfate and evaporated to dryness. The
residue is recrystallized from a mixture of ether and petroleum ether, giving
δ4-17β-hydroxy-17α-allyl-oestrene, melting point 79.5°-80°C.
Therapeutic Function
Progestin; Antiandrogen
Properties of Allylestrenol
| Melting point: | 79.5-80° |
| Boiling point: | 381.7°C (rough estimate) |
| Density | 0.9914 (rough estimate) |
| refractive index | 1.4800 (estimate) |
| storage temp. | -20°C Freezer |
| solubility | Chloroform (Slightly), Methanol (Slightly) |
| form | Solid |
| pka | 14.95±0.40(Predicted) |
| color | White to Pale Yellow |
| Merck | 14,291 |
| CAS DataBase Reference | 432-60-0(CAS DataBase Reference) |
Safety information for Allylestrenol
| Signal word | Warning |
| Pictogram(s) |
 Exclamation Mark Irritant GHS07 |
| GHS Hazard Statements |
H315:Skin corrosion/irritation H319:Serious eye damage/eye irritation H335:Specific target organ toxicity, single exposure;Respiratory tract irritation |
| Precautionary Statement Codes |
P261:Avoid breathing dust/fume/gas/mist/vapours/spray. P305+P351+P338:IF IN EYES: Rinse cautiously with water for several minutes. Remove contact lenses, if present and easy to do. Continuerinsing. |
Computed Descriptors for Allylestrenol
New Products
4,4-Difluoropiperidine hydrochloride tert-butyl 9-methoxy-3-azaspiro[5.5]undecane-3-carboxylate Indole Methyl Resin N-Isopropylurea N,N-Dicyclohexylcarbodiimide(DCC) MELDRUMS ACID 5-METHYLISOXAZOLE-4-CARBOXYLIC ACID Magnessium Bis glycinate Zinc ascorbate 1-bromo-2-butyne 2-acetamidophenol 9(10H)-anthracenone Erythrosin B, 4-Piperidinopiperidine 2-((4-morpholinophenylamino) (methylthio) methylene) malononitrile 2,4-dihydroxybenzaldehyde 3-(4-morpholinophenylamino)-5-amino-1H-pyrazole-4-carbonitrile Methyl 2-methylquinoline-6-carboxylate 2,6-dichloro-4-nitropyridine 4-Bromo-2-chlorobenzonitrile 2-(benzylamino)acetic acid hydrochloride 4-(tert-Butoxycarbonylamino)but- 2-ynoic acid 3,4-dihydro-2H-benzo[b][1,4]dioxepine 1-Phenyl-1-cycloprppanecarboxylicacidRelated products of tetrahydrofuran




You may like
-
 Allylestrenol 99%View Details
Allylestrenol 99%View Details -
 432-60-0 98%View Details
432-60-0 98%View Details
432-60-0 -
 Allyloestrenol 98%View Details
Allyloestrenol 98%View Details
432-60-0 -
 Allyloestrenol 432-60-0 98%View Details
Allyloestrenol 432-60-0 98%View Details
432-60-0 -
 432-60-0 Allyloestrenol 99%View Details
432-60-0 Allyloestrenol 99%View Details
432-60-0 -
 Allylestrenol 95% CAS 432-60-0View Details
Allylestrenol 95% CAS 432-60-0View Details
432-60-0 -
 Allylestrenol API powderView Details
Allylestrenol API powderView Details
432-60-0 -
 Medicine Grade Allylestrenol CAS No: 432-60-0View Details
Medicine Grade Allylestrenol CAS No: 432-60-0View Details
432-60-0
