Almotriptan
- CAS NO.:154323-57-6
- Empirical Formula: C17H25N3O2S
- Molecular Weight: 335.46
- MDL number: MFCD00927104
- EINECS: 1312995-182-4
- SAFETY DATA SHEET (SDS)
- Update Date: 2024-11-19 23:02:33
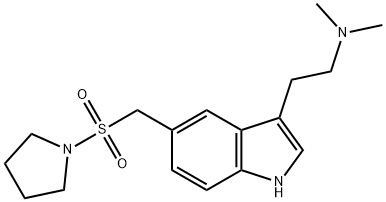
What is Almotriptan?
Description
Almotriptan was first marketed in Spain as a new medicine against acute attacks of migraine. It is the fifth agent belonging to the “triptan” class to be launched after sumatriptan, naratriptan, zolmitriptan and rizatriptan. This close structural analog of sumatriptan can be prepared in six steps from 4-nitrobenzylsulfonyl chloride with a Fischer indole synthesis as the key step. Almotriptan acts as a dual 5-HT1D/1B agonist with a 35 to 51-fold selectivity versus 5-HT1A and 5-HT7 receptors respectively as well as having insignificant affinity for the most relevant nonserotonergic receptors (K1>1μM). Its agonistic effect on 5-HT,n receptors of trigeminal sensory neurons turns off neurogenic inflammation by inhibiting the release of neuropeptides such as calcitonin gene-related peptide, neurokinin A and substance P. Concomitantly, its action on the 5-HT1B receptors in meningeal arteries relieves the vasodilatation of these vessels associated with migraine attacks. Almotriptan causes selective concentration-dependent vasoconstriction of human meningeal and temporal arteries (with EC50 of 0.03 and 0.7 μM) compared to basilar (EC50 = 3.5 μM) and pulmonary arteries (EC50>10μM) or rabbit mesenteric and renal arteries (EC50>100 μM). Although it is predominantly cleared by the kidneys as unchanged drug (45%) or transformed into inactive metabolites by monoamine oxidase A (MAO-A) and CYP3A4 enzymes in the liver, almotriptan has the highest oral bioavailability (70%) of the triptans and has a half-life of 3.5 h. The therapeutic dose of 12.5 mg is well tolerated, shows a rapid onset of action (30 min) and low recurrence rate compared to sumatriptan.
Originator
Almirall Prodesfarma (Spain)
The Uses of Almotriptan
Serotonin 5HT1B /1D-receptor agonist
Background
Almotriptan is a triptan drug for the treatment of migraine headaches. Almotriptan is in a class of medications called selective serotonin receptor agonists. It works by narrowing blood vessels in the brain, stopping pain signals from being sent to the brain, and stopping the release of certain natural substances that cause pain, nausea, and other symptoms of migraine. Almotriptan does not prevent migraine attacks.
Indications
For the treatment of acute migraine headache in adults
Definition
ChEBI: An indole compound having a 2-(dimethylamino)ethyl group at the 3-position and a (pyrrolidin-1-ylsulfonyl)methyl group at the 5-position.
Manufacturing Process
To a solution of previously dried 1-[[2-carboxy-3-(2-dimethylaminoethyl)-5-
indolyl]methanesulphonyl]-pyrrolidine (1.6 g; 0.0442 moles) in anhydrous
quinoline (75 ml) and under atmosphere of nitrogen, cuprous oxide (160 mg;
0.0011 moles) was added. The reaction mixture was heated to 190°C for 15
minutes, stirred to room temperature, poured into a mixture of 1 N
hydrochloric acid (150 ml) and ethyl acetate (50 ml), shaken and decanted.
The aqueous solution was washed several times with ethyl acetate, then solid
sodium bicarbonate was added until pH = 7.8, and washed with n-hexane to
eliminate the quinoline. The aqueous solution was made alkaline with solid
potassium carbonate and extracted with ethyl acetate. The organic solution
was dried (Na2SO4), the solvent removed under reduced pressure when a
dark oil was obtained (1.3 g; yield 92%). This product was purified by column
chromatography with silica gel and methylene chloride:ethanol:ammonium
hydroxide (60:8:1) as eluent and a white foam (0.8 g) of 1-[[3-(2-
dimethylaminoethyl)-5-indolyl]methanesulphonyl]-pyrrolidine was obtained. To
a solution of the above product (0.8 g) in acetone (30 ml), a few drops of
hydrogen chloride saturated dioxan solution, were added. The precipitated
solid was collected by filtration, washed with acetone and dried to give 1-[(3-
(2-(dimethylamino)ethyl)-5-indolyl)methanesulphonyl]-pyrrolidine
hydrochloride (0.75 g). Melting point 218°-220°C.
In practice it is usually used as malate salt.
brand name
Almogran
Therapeutic Function
Migraine therapy
Pharmacokinetics
Almotriptan is a selective 5-hydroxytryptamine receptor subtype agonist indicated for the acute treatment of migraine attacks with or without aura in adults. Almotriptan is not intended for the prophylactic therapy of migraine or for use in the management of hemiplegic or basilar migraine. Almotriptan is an agonist for a vascular 5-hydroxytryptamine receptor subtype (probably a member of the 5-HT1D family) having only a weak affinity for 5-HT1A, 5-HT5A, and 5-HT7 receptors and no significant affinity or pharmacological activity at 5-HT2, 5-HT3 or 5-HT4 receptor subtypes or at alpha1-, alpha2-, or beta-adrenergic, dopamine1,; dopamine2; muscarinic, or benzodiazepine receptors. This action in humans correlates with the relief of migraine headache. In addition to causing vasoconstriction, experimental data from animal studies show that Almotriptan also activates 5-HT1 receptors on peripheral terminals of the trigeminal nerve innervating cranial blood vessels, which may also contribute to the antimigrainous effect of Almotriptan in humans.
Clinical Use
5HT1
receptor agonist:
Acute relief of migraine
Drug interactions
Potentially hazardous interactions with other drugs
Antidepressants: increased risk of CNS toxicity with
citalopram - avoid; possibly increased serotonergic
effects with duloxetine or venlafaxine; increased
serotonergic effects with St John’s wort - avoid.
Antifungals: concentration increased by ketoconazole
(increased risk of toxicity).
Dapoxetine: possible increased risk of serotonergic
effects - avoid for 2 weeks after stopping 5HT1
agonists.
Ergot alkaloids: increased risk of vasospasm - avoid.
Metabolism
Metabolism
The major biotransformation route is via monoamine oxidase (MAO-A) mediated oxidative deamination to the indole acetic metabolite. Cytochrome P450 (3A4 and 2D6 isozymes) and flavin mono-oxygenase are other enzymes involved in the metabolism of almotriptan. None of the metabolites are significantly active pharmacologically. More than 75% of a dose is eliminated in urine, and the remainder in faeces. Approximately, 50% of the urinary and faecal excretion is unchanged almotriptan.
Properties of Almotriptan
| Boiling point: | 538.7±60.0 °C(Predicted) |
| Density | 1.27±0.1 g/cm3(Predicted) |
| pka | 16.92±0.30(Predicted) |
| form | Solid |
| color | White to off-white |
| CAS DataBase Reference | 154323-57-6(CAS DataBase Reference) |
Safety information for Almotriptan
| Signal word | Warning |
| Pictogram(s) |
 Exclamation Mark Irritant GHS07 |
| GHS Hazard Statements |
H315:Skin corrosion/irritation H319:Serious eye damage/eye irritation H335:Specific target organ toxicity, single exposure;Respiratory tract irritation |
| Precautionary Statement Codes |
P233:Keep container tightly closed. P260:Do not breathe dust/fume/gas/mist/vapours/spray. P261:Avoid breathing dust/fume/gas/mist/vapours/spray. P264:Wash hands thoroughly after handling. P264:Wash skin thouroughly after handling. P270:Do not eat, drink or smoke when using this product. P271:Use only outdoors or in a well-ventilated area. P280:Wear protective gloves/protective clothing/eye protection/face protection. P304:IF INHALED: P312:Call a POISON CENTER or doctor/physician if you feel unwell. P321:Specific treatment (see … on this label). P330:Rinse mouth. P340:Remove victim to fresh air and keep at rest in a position comfortable for breathing. P362:Take off contaminated clothing and wash before reuse. P301+P312:IF SWALLOWED: call a POISON CENTER or doctor/physician IF you feel unwell. P302+P352:IF ON SKIN: wash with plenty of soap and water. P304+P340:IF INHALED: Remove victim to fresh air and Keep at rest in a position comfortable for breathing. P305+P351+P338:IF IN EYES: Rinse cautiously with water for several minutes. Remove contact lenses, if present and easy to do. Continuerinsing. P332+P313:IF SKIN irritation occurs: Get medical advice/attention. P337+P313:IF eye irritation persists: Get medical advice/attention. P403:Store in a well-ventilated place. P405:Store locked up. P403+P233:Store in a well-ventilated place. Keep container tightly closed. P501:Dispose of contents/container to..… |
Computed Descriptors for Almotriptan
Abamectin manufacturer
Vepan Pharmatech Pvt Ltd
New Products
4-Aminotetrahydropyran-4-carbonitrile Hydrochloride (R)-3-Aminobutanenitrile Hydrochloride 4-AMINO-TETRAHYDRO-PYRAN-4-CARBOXYLIC ACID HCL 4-(Dimethylamino)tetrahydro-2H-pyran-4-carbonitrile 3-((Dimethylamino)methyl)-5-methylhexan-2-one oxalate 1,4-Dioxa-8-azaspiro[4.5]decane 5-Bromo-2-nitropyridine Nimesulide BP Aceclofenac IP/BP/EP Diclofenac Sodium IP/BP/EP/USP Mefenamic Acid IP/BP/EP/USP Ornidazole IP Diclofenac Potassium SODIUM AAS SOLUTION ZINC AAS SOLUTION BUFFER SOLUTION PH 10.0(BORATE) GOOCH CRUCIBLE SINTERED AQUANIL 5 BERYLLIUM AAS SOLUTION 2-Bromo-1-(bromomethyl)-3-chloro-5-nitrobenzene 2-Bromo-3-nitroaniline N-(3-Hydroxypropyl)-N-methylacetamide 3-Bromo-6-chloropyridazine 4-ethyl-3-nitrobenzoic acidRelated products of tetrahydrofuran


You may like
-
 Almotriptan 98%View Details
Almotriptan 98%View Details -
 154323-57-6 99%View Details
154323-57-6 99%View Details
154323-57-6 -
 Almotriptan 98%View Details
Almotriptan 98%View Details
154323-57-6 -
![1-[[[3-[2- (Dimethylamino) ethyl]- 1H- indol-yl] sulfonyl] pyrrolidine - Base](https://img.chemicalbook.in//ProductImageIndia/2024-09/Raw/eb20a9da-0fa8-4345-a7ba-94127507d1cc.jpg) 1-[[[3-[2- (Dimethylamino) ethyl]- 1H- indol-yl] sulfonyl] pyrrolidine - BaseView Details
1-[[[3-[2- (Dimethylamino) ethyl]- 1H- indol-yl] sulfonyl] pyrrolidine - BaseView Details
154323-57-6 -
 1823368-42-8 98%View Details
1823368-42-8 98%View Details
1823368-42-8 -
 2-(3-(tert-butyl)phenoxy)-2-methylpropanoic acid 1307449-08-6 98%View Details
2-(3-(tert-butyl)phenoxy)-2-methylpropanoic acid 1307449-08-6 98%View Details
1307449-08-6 -
 Ethyl 3-(furan-2-yl)-3-hydroxypropanoate 25408-95-1 98%View Details
Ethyl 3-(furan-2-yl)-3-hydroxypropanoate 25408-95-1 98%View Details
25408-95-1 -
 Lithium ClavulanateView Details
Lithium ClavulanateView Details
61177-44-4