Adamantane
Synonym(s):Adamantane;Tricyclo[3.3.1.1(3,7)]decane
- CAS NO.:281-23-2
- Empirical Formula: C10H16
- Molecular Weight: 136.23
- MDL number: MFCD00074719
- EINECS: 206-001-4
- SAFETY DATA SHEET (SDS)
- Update Date: 2025-12-15 16:23:17
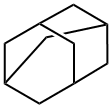
What is Adamantane?
Description
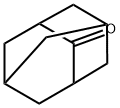
Adamantane (formally tricyclo[3.3.1.13,7]decane) is an exquisitely symmetrical hydrocarbon that was originally isolated from petroleum in 1933 by the Czech researchers S. Landa and V. Machacek. Its synthesis was first reported by Vladimir Prelog in 1941. It’s the simplest diamondoid compound and is nice to look at, but it has few practical applications.
Chemical properties
slightly beige crystals
The Uses of Adamantane
Adamantane is mainly used for the synthesis of medicine to treat influenza, serve as NMDA receptor channel blockers. Also be used for preparation of advanced lubricants, photographic material, surface active agents, catalysts and other products.
The Uses of Adamantane
synthon for Amantadine, Rimantadine, Somantadine, Tromantadine
The Uses of Adamantane
The diamine as curing agent for epoxy resins [US 3053907 (1962 to du Pont)].
Definition
adamantane: A colourless crystallinehydrocarbon C10H16; m.p.269°C. It is found in certain petroleumfractions. The structure containsthree symmetrically fusedcyclohexane rings.
Synthesis Reference(s)
Journal of the American Chemical Society, 91, p. 6779, 1969 DOI: 10.1021/ja01052a041
The Journal of Organic Chemistry, 51, p. 3038, 1986 DOI: 10.1021/jo00365a034
Purification Methods
Crystallise adamantane from acetone or cyclohexane, and sublime it in a vacuum below its melting point [Butler et al. J Chem Soc, Faraday Trans I 82 535 1986]. Adamantane is also purified by dissolving it in n-heptane (ca 10mL/g of adamantane) on a hot plate, adding activated charcoal (2g/100g of adamantane), and boiling for 30minutes, filtering the hot solution through a filter paper, concentrating the filtrate until crystallisation just starts, adding one quarter of the original volume of n-heptane, and allowing to cool slowly over a period of hours. The supernatant is decanted off and the crystals are dried in vacuo at 25o. [Prelog & Seiwerth Chem Ber 74 1769 1941, Schleyer et al. Org Synth Coll Vol V 16 1973, Walter et al. J Am Chem Soc 107 793 1985.] [Beilstein 5 III 393, 5 IV 469.]
Properties of Adamantane
| Melting point: | 209-212 °C (subl.) (lit.) |
| Boiling point: | 185.55°C (rough estimate) |
| Density | 1,07 g/cm3 |
| refractive index | 1.5680 |
| storage temp. | Store below +30°C. |
| solubility | 0.00021g/l slightly soluble |
| form | Crystals or Crystalline Powder |
| Specific Gravity | 1.07 |
| color | White to beige |
| Water Solubility | Insoluble in water. |
| Merck | 14,149 |
| BRN | 1901173 |
| Stability: | Stable. Combustible. Incompatible with strong oxidizing agents. |
| CAS DataBase Reference | 281-23-2(CAS DataBase Reference) |
| NIST Chemistry Reference | Adamantane(281-23-2) |
| EPA Substance Registry System | Tricyclo[3.3.1.13,7]decane (281-23-2) |
Safety information for Adamantane
| Signal word | Warning |
| Pictogram(s) |
 Environment GHS09 |
| GHS Hazard Statements |
H413:Hazardous to the aquatic environment, long-term hazard |
| Precautionary Statement Codes |
P273:Avoid release to the environment. |
Computed Descriptors for Adamantane
| InChIKey | ORILYTVJVMAKLC-UHFFFAOYSA-N |
Adamantane manufacturer
New Products
4,4-Difluoropiperidine hydrochloride tert-butyl 9-methoxy-3-azaspiro[5.5]undecane-3-carboxylate Indole Methyl Resin N-Isopropylurea N,N-Dicyclohexylcarbodiimide(DCC) MELDRUMS ACID 5-METHYLISOXAZOLE-4-CARBOXYLIC ACID Magnessium Bis glycinate Zinc ascorbate 1-bromo-2-butyne 2-acetamidophenol 9(10H)-anthracenone Erythrosin B, 4-Piperidinopiperidine 2-((4-morpholinophenylamino) (methylthio) methylene) malononitrile 2,4-dihydroxybenzaldehyde 3-(4-morpholinophenylamino)-5-amino-1H-pyrazole-4-carbonitrile Methyl 2-methylquinoline-6-carboxylate 2,6-dichloro-4-nitropyridine 4-Bromo-2-chlorobenzonitrile 2-(benzylamino)acetic acid hydrochloride 4-(tert-Butoxycarbonylamino)but- 2-ynoic acid 3,4-dihydro-2H-benzo[b][1,4]dioxepine 1-Phenyl-1-cycloprppanecarboxylicacidRelated products of tetrahydrofuran




You may like
-
 281-23-2 98%View Details
281-23-2 98%View Details
281-23-2 -
 281-23-2 Adamantane 98%View Details
281-23-2 Adamantane 98%View Details
281-23-2 -
 Adamantane (purified by sublimation) CAS 281-23-2View Details
Adamantane (purified by sublimation) CAS 281-23-2View Details
281-23-2 -
 Adamantane CAS 281-23-2View Details
Adamantane CAS 281-23-2View Details
281-23-2 -
 Adamantane, 99% CAS 281-23-2View Details
Adamantane, 99% CAS 281-23-2View Details
281-23-2 -
 Adamantane 95% CAS 281-23-2View Details
Adamantane 95% CAS 281-23-2View Details
281-23-2 -
 Adamantane CAS 281-23-2View Details
Adamantane CAS 281-23-2View Details
281-23-2 -
 Adamantane >99% (GC) CAS 281-23-2View Details
Adamantane >99% (GC) CAS 281-23-2View Details
281-23-2
