Phenylpropyl aldehyde
Synonym(s):3-Phenylpropanal;3-Phenylpropionaldehyde;Hydrocinnamaldehyde, 3-Phenylpropanal
- CAS NO.:104-53-0
- Empirical Formula: C9H10O
- Molecular Weight: 134.18
- MDL number: MFCD00007021
- EINECS: 203-211-8
- SAFETY DATA SHEET (SDS)
- Update Date: 2025-12-20 21:15:22
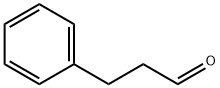
What is Phenylpropyl aldehyde?
Chemical properties
Pale Yellow Oil
Chemical properties
Phenylpropyl aldehyde occurs in Sri Lanka cinnamon bark oil, among others. The aldehyde is a colorless liquid with a strong, floral, slightly balsamic, heavy hyacinth-like odor. It tends to undergo self-condensation. Dihydrocinnamaldehyde can be obtained with scarcely any byproducts by selective hydrogenation of cinnamaldehyde. It is used in perfumery for hyacinth and lilac compositions.
Chemical properties
3-Phenylpropionaldehyde has a strong, pungent, floral odor reminiscent of hyacinth with a balsamic, green, warm (almost burning) flavor.
Occurrence
Reported found in the essential oil of Ceylon cinnamon and in strawberry. Also reported found in tomato, cinnamon, cassia leaf, Gruyere de Comte cheese, beer, cooked trassi, origanum (Spanish) and strawberry.
The Uses of Phenylpropyl aldehyde
3-Phenylpropanal (cas# 104-53-0) is a compound useful in organic synthesis.
Preparation
From phenyl propionitrile; also from cinnamic aldehyde diethylacetal.
Definition
ChEBI: 3-phenylpropanal is a benzene which is substituted by a 3-oxopropyl group at position 1. It has a role as a flavouring agent and a plant metabolite. It is an aldehyde and a member of benzenes. It is functionally related to a benzene.
Production Methods
Henylpropyl aldehyde is most commonly prepared by the partial hydrogenation of cinnamaldehyde. The 3-phenylpropanal that is formed during the hydroformylation of styrene can be separated from the isomeric 2-phenylpropanal as the bisulfite adduct. The Rosenmund reduction of dihydrocinnamoyl chloride in the presence of Pd/BaSO4 gives good yields of the aldehyde. The reaction of 2-propen-1-ol and phenylmercury chloride with CuCl2 and LiPdCl4 catalysts in methanol, and the reaction of phenyl bromide and allyl alcohol in the presence of PdCl2 and NaHCO3 in nonpolar solvents also lead to the formation of 3-phenylpropanal.
Taste threshold values
Taste characteristics at 20 ppm: green, melon, fruity and citrus.
Synthesis Reference(s)
Chemical and Pharmaceutical Bulletin, 42, p. 1041, 1994 DOI: 10.1248/cpb.42.1041
Tetrahedron Letters, 35, p. 1275, 1994 DOI: 10.1016/0040-4039(94)88042-5
General Description
Hydrocinnamaldehyde is C=C double bond hydrogenated cinnamaldehyde. It has been synthesized by the selective hydrogenation of C=C double bond of cinnamaldehyde by various methods. Hydrocinnamaldehyde and nitromethane undergoes Henry reaction to form nitroaldols. The C1 and C3 position labelled with 13C of hydrocinnamaldehyde was subjected to mass spectrometry and the fragmentation pattern was elucidated.
Properties of Phenylpropyl aldehyde
| Melting point: | -42 °C |
| Boiling point: | 97-98 °C/12 mmHg (lit.) |
| Density | 1.019 g/mL at 25 °C (lit.) |
| vapor pressure | 15 hPa (98 °C) |
| FEMA | 2887 | 3-PHENYLPROPIONALDEHYDE |
| refractive index | n |
| Flash point: | 203 °F |
| storage temp. | Store below +30°C. |
| solubility | 0.74mg/l |
| form | Liquid |
| color | Clear colorless to light yellow |
| Odor | at 10.00 % in dipropylene glycol. fresh cortex green leaf foliage balsam storax |
| Water Solubility | Miscible with chloroform, dichloromethane, ethyl acetate, alcohol and ether. Immiscible with water. |
| Sensitive | Air Sensitive |
| JECFA Number | 645 |
| BRN | 1071910 |
| CAS DataBase Reference | 104-53-0(CAS DataBase Reference) |
| NIST Chemistry Reference | 3-Phenylpropanal(104-53-0) |
| EPA Substance Registry System | Benzenepropanal (104-53-0) |
Safety information for Phenylpropyl aldehyde
| Signal word | Warning |
| Pictogram(s) |
 Exclamation Mark Irritant GHS07 |
| GHS Hazard Statements |
H315:Skin corrosion/irritation H319:Serious eye damage/eye irritation H335:Specific target organ toxicity, single exposure;Respiratory tract irritation |
| Precautionary Statement Codes |
P261:Avoid breathing dust/fume/gas/mist/vapours/spray. P304+P340:IF INHALED: Remove victim to fresh air and Keep at rest in a position comfortable for breathing. P305+P351+P338:IF IN EYES: Rinse cautiously with water for several minutes. Remove contact lenses, if present and easy to do. Continuerinsing. P405:Store locked up. |
Computed Descriptors for Phenylpropyl aldehyde
| InChIKey | YGCZTXZTJXYWCO-UHFFFAOYSA-N |
New Products
Indole Methyl Resin tert-butyl 9-methoxy-3-azaspiro[5.5]undecane-3-carboxylate Boc-His(Boc)-OH 2-CTC Resin 4-Chloro-7-tosy1-7Hpyrrolo[2,3-d]pyrimidine 5,7-Dibromo-1H-indole 2,5-dichloro-N-hydroxy-4,6-dimethylpyridine-3-carboximidamide 2,2-Dimethoxy-7-azaspiro[3.5]nonane hydrochloride 4-chloromethyl-5-methyl-1,3-dioxol-2-one (DMDO-Cl) R-2-BENZYLOXY PROPIONIC ACID 1,1’-CARBONYLDIIMIDAZOLE 1,1’-CARBONYLDI (1,2-4 TRIAZOLE) N-METHYL INDAZOLE-3-CARBOXYLIC ACID 4-((2-hydroxyethyl)thio)benzoic acid 1-(TERT-BUTOXYCARBONYL)-2-PYRROLIDINONE Methyl 6-methylnicotinate 3-Pyridineacrylic acid tert-Butyl carbazate TETRAHYDRO-2H-PYRAN-3-OL 2-((4-morpholinophenylamino) (methylthio) methylene) malononitrile 3-(4-morpholinophenylamino)-5-amino-1H-pyrazole-4-carbonitrile 2,4-dihydroxybenzaldehyde 1,3-Diethyl-1,3-Diphenylurea Methyl 2-methylquinoline-6-carboxylateRelated products of tetrahydrofuran


You may like
-
 104-53-0 3-Phenylpropionaldehyde 98%View Details
104-53-0 3-Phenylpropionaldehyde 98%View Details
104-53-0 -
 104-53-0 98%View Details
104-53-0 98%View Details
104-53-0 -
 Phenylpropyl aldehyde 95.00% CAS 104-53-0View Details
Phenylpropyl aldehyde 95.00% CAS 104-53-0View Details
104-53-0 -
 3-Phenylpropionaldehyde CAS 104-53-0View Details
3-Phenylpropionaldehyde CAS 104-53-0View Details
104-53-0 -
 Hydrocinnamaldehyde CAS 104-53-0View Details
Hydrocinnamaldehyde CAS 104-53-0View Details
104-53-0 -
 3-Phenylpropionaldehyde CAS 104-53-0View Details
3-Phenylpropionaldehyde CAS 104-53-0View Details
104-53-0 -
 Phenylpropyl Aldehyde CAS 104-53-0View Details
Phenylpropyl Aldehyde CAS 104-53-0View Details
104-53-0 -
 Thiourea 99% ARView Details
Thiourea 99% ARView Details
62-56-6
