Acridine
- CAS NO.:260-94-6
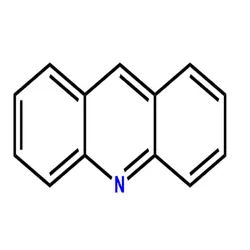
- Empirical Formula: C13H9N
- Molecular Weight: 179.22
- MDL number: MFCD00005025
- EINECS: 205-971-6
- SAFETY DATA SHEET (SDS)
- Update Date: 2025-09-25 17:15:13
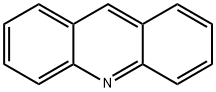
What is Acridine?
Chemical properties
colourless to light yellow crystals
The Uses of Acridine
manufacture of dyes and intermediates; some dyes derived from it are used as antiseptics, e.g. 9-aminoacridine, acriflavine and proflavine. The hydrochloride has been used as reagent for cobalt, iron and zinc.
The Uses of Acridine
A quinoline derivative used as manufacturing dyes and as intermediate for antileishmanial agents. A catabolic product of carbamazepine (C175840) metabolite.
The Uses of Acridine
Acridine is a compound occurring in coal tar that has been used in the manufacture of dyes and intermediates. Derivatives are used as antiseptics (e.g., proflavine). Acridine is a strong irritant to mucous membranes and skin, and it causes sneezing on inhalation.
What are the applications of Application
Acridine is a useful dye for biological research purposes
Definition
ChEBI: A polycyclic heteroarene that is anthracene in which one of the central CH groups is replaced by a nitrogen atom.
Definition
A colorless crystalline heterocyclic compound with three fused rings. Derivatives of acridine are used as dyes and biological stains.
Definition
acridine: A colourless crystallineheterocyclic compound, C12H9N; m.p.110°C. The ring structure is similarto that of anthracene, with threefused rings, the centre ring containinga nitrogen heteroatom. Severalderivatives of acridine (such as acridineorange) are used as dyes or biologicalstains.
brand name
Euflavin;Proflavin.
World Health Organization (WHO)
Acridine derivatives with antiseptic and disinfectant activity, including acriflavine, proflavine and euflavine, were formerly used in the treatment of infected wounds and burns. Such use has largely been discontinued on the grounds that safer and more effective alternatives are now available. Following demonstration of the mutagenic activity of proflavine in 1978 it was withdrawn from dental products in Denmark. Subsequently, euflavine was similarly withdrawn.
Synthesis Reference(s)
The Journal of Organic Chemistry, 21, p. 1286, 1956 DOI: 10.1021/jo01117a019
Synthesis, p. 732, 1987 DOI: 10.1055/s-1987-28066
General Description
Small colorless needle-like crystalline solid. Slightly soluble in hot water. Slightly denser than water. Contact may irritate skin, eyes, and mucous membranes. Sublimes before melting when heated. May be toxic by ingestion.
Air & Water Reactions
Slightly soluble in hot water.
Reactivity Profile
Acridine neutralizes acids in exothermic reactions to form salts plus water. May be incompatible with isocyanates, halogenated organics, peroxides, phenols (acidic), epoxides, anhydrides, and acid halides. Flammable gaseous hydrogen may be generated in combination with strong reducing agents, such as hydrides. Burns to give toxic oxides of nitrogen.
Health Hazard
Inhalation irritates respiratory system and causes sneezing, crying, and vomiting. Contact with liquid irritates eyes, skin, and mucous membranes. At high temperature and during sun exposure, damage to the cornea, skin, and mucous membranes may occur following the liberation of Acridine vapor.
Safety Profile
Poison by ingestion, subcutaneous, and intravenous routes. Mutation data reported. A skin, eye, and mucous membrane irritant. When heated to decomposition it emits toxic fumes of NO,.
Potential Exposure
Acridine and its derivatives are widely used in the production of dyestuffs, such as acriflavine, benzoflavine, and chrysaniline; and in the synthesis of pharmaceuticals; such as aurinacrine, proflavine, and rivanol. A constituent of coal tar, coal tar creosote; found in wastes from gas and tar plants and coke oven emissions. Incompatibilities: Strong acids, strong oxidizers.
Shipping
UN2713 Acridine, Hazard Class: 6.1; Labels: 6.1-Poisonous materials
Purification Methods
Acridine has been crystallised twice from *benzene/cyclohexane, or from aqueous EtOH, then sublimed, removing and discarding the first 25% of the sublimate. The remainder is again crystallised and sublimed, discarding the first 10-15% [Wolf & Anderson J Am Chem Soc 77 1608 1955]. Acridine can also be purified by crystallisation from n-heptane and then from ethanol/water after pre-treatment with activated charcoal, or by chromatography on alumina with pet ether in a darkened room. Alternatively, acridine can be precipitated as the hydrochloride from *benzene solution by adding HCl, after which the base is regenerated, dried at 110o/50mm, and recrystallised to constant melting point from pet ether [Cumper et al. J Chem Soc 4518 1962]. The regenerated free base may be recrystallised, chromatographed on basic alumina, then vacuum-sublimed and zone-refined. [Williams & Clarke, J Chem Soc, Faraday Trans 1 73 514 1977, Albert, The Acridines Arnold Press 1966.] It can exist in five crystalline forms and is steam volatile. It is a strong IRRITANT to skin and mucous membranes and can become a chronic irritant— handle it with CARE. [Beilstein 20/8 V 199.]
Incompatibilities
Acridine and its derivatives are widely used in the production of dyestuffs, such as acriflavine, benzoflavine, and chrysaniline; and in the synthesis of pharmaceuticals; such as aurinacrine, proflavine, and rivanol. A constituent of coal tar, coal tar creosote; found in wastes from gas and tar plants and coke oven emissions. Incompatibilities: Strong acids, strong oxidizers.
Waste Disposal
Incineration with nitrogen oxide removal from the effluent gas by scrubber, catalytic, or thermal device.
Properties of Acridine
| Melting point: | 106-109 °C |
| Boiling point: | 346 °C(lit.) |
| Density | 1,005 g/cm3 |
| refractive index | 1.7270 (estimate) |
| Flash point: | 346°C |
| storage temp. | Refrigerator |
| solubility | dioxane: 0.1 g/mL, clear |
| form | Crystalline Powder |
| pka | 5.58(at 20℃) |
| color | Yellow to yellow-brown |
| PH Range | Green B uorescence (5.2) to violet B uorescence (6.6) |
| Water Solubility | 57.35mg/L(24 ºC) |
| λmax | 358nm, 392nm |
| Merck | 14,122 |
| BRN | 120200 |
| Exposure limits | OSHA: TWA 0.2 mg/m3 |
| Stability: | Stable. Combustible. Incompatible with strong oxidizing agents. |
| Major Application | Organic light-emitting diode, photoresists, nanosensors, fuel cells, memory device, semiconductors, information storage device, liquid crystal displays, identification of product forgeries, inks, adhesives, textile, hair dyes, gene detection assay, staining cells, biosensors, detecting nucleic acids, proteins, antiHCV antibodies, antibacterial, antiamyloid agents, wound dressing materials, vaccines against infection, allergy and cancer |
| CAS DataBase Reference | 260-94-6(CAS DataBase Reference) |
| EPA Substance Registry System | Acridine (260-94-6) |
Safety information for Acridine
| Signal word | Warning |
| Pictogram(s) |
 Exclamation Mark Irritant GHS07  Environment GHS09 |
| GHS Hazard Statements |
H302:Acute toxicity,oral H315:Skin corrosion/irritation H319:Serious eye damage/eye irritation H335:Specific target organ toxicity, single exposure;Respiratory tract irritation H410:Hazardous to the aquatic environment, long-term hazard |
| Precautionary Statement Codes |
P261:Avoid breathing dust/fume/gas/mist/vapours/spray. P264:Wash hands thoroughly after handling. P264:Wash skin thouroughly after handling. P273:Avoid release to the environment. P301+P312:IF SWALLOWED: call a POISON CENTER or doctor/physician IF you feel unwell. P302+P352:IF ON SKIN: wash with plenty of soap and water. P305+P351+P338:IF IN EYES: Rinse cautiously with water for several minutes. Remove contact lenses, if present and easy to do. Continuerinsing. |
Computed Descriptors for Acridine
| InChIKey | DZBUGLKDJFMEHC-UHFFFAOYSA-N |
Acridine manufacturer
New Products
Indole Methyl Resin tert-butyl 9-methoxy-3-azaspiro[5.5]undecane-3-carboxylate Boc-His(Boc)-OH 2-CTC Resin 4-Chloro-7-tosy1-7Hpyrrolo[2,3-d]pyrimidine 5,7-Dibromo-1H-indole 2,5-dichloro-N-hydroxy-4,6-dimethylpyridine-3-carboximidamide 2,2-Dimethoxy-7-azaspiro[3.5]nonane hydrochloride 4-chloromethyl-5-methyl-1,3-dioxol-2-one (DMDO-Cl) R-2-BENZYLOXY PROPIONIC ACID 1,1’-CARBONYLDIIMIDAZOLE 1,1’-CARBONYLDI (1,2-4 TRIAZOLE) N-METHYL INDAZOLE-3-CARBOXYLIC ACID 4-((2-hydroxyethyl)thio)benzoic acid 1-(TERT-BUTOXYCARBONYL)-2-PYRROLIDINONE Methyl 6-methylnicotinate 3-Pyridineacrylic acid tert-Butyl carbazate TETRAHYDRO-2H-PYRAN-3-OL 2-((4-morpholinophenylamino) (methylthio) methylene) malononitrile 3-(4-morpholinophenylamino)-5-amino-1H-pyrazole-4-carbonitrile 2,4-dihydroxybenzaldehyde 1,3-Diethyl-1,3-Diphenylurea Methyl 2-methylquinoline-6-carboxylateRelated products of tetrahydrofuran


You may like
-
 260-94-6 98%View Details
260-94-6 98%View Details
260-94-6 -
 Acridine 95% CAS 260-94-6View Details
Acridine 95% CAS 260-94-6View Details
260-94-6 -
 Acridine 98.00% CAS 260-94-6View Details
Acridine 98.00% CAS 260-94-6View Details
260-94-6 -
 Acridine (purified by sublimation) CAS 260-94-6View Details
Acridine (purified by sublimation) CAS 260-94-6View Details
260-94-6 -
 Acridine CAS 260-94-6View Details
Acridine CAS 260-94-6View Details
260-94-6 -
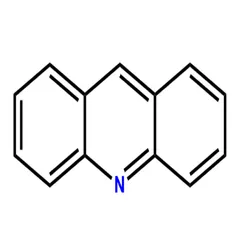 AcridineView Details
AcridineView Details
260-94-6 -
 White Acridine ChemicalView Details
White Acridine ChemicalView Details
260-94-6 -
 AcridineView Details
AcridineView Details
260-94-6
