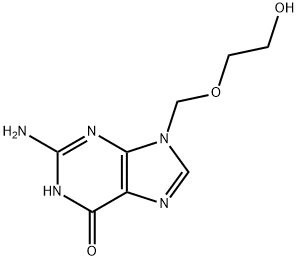
Acyclovir sodium
- CAS NO.:69657-51-8
- Empirical Formula: C8H12N5NaO3
- Molecular Weight: 249.21
- MDL number: MFCD01694138
- EINECS: 614-996-5
- SAFETY DATA SHEET (SDS)
- Update Date: 2025-12-11 08:41:34

What is Acyclovir sodium?
Chemical properties
Acyclovir sodium is a white, crystalline powder with the molecular formula C8H12N5NaO3. The maximum solubility in water at 25°C exceeds 100 mg/mL. At physiologic pH, acyclovir sodium exists as the unionized form with a molecular weight of 225 and a maximum solubility in water at 37°C of 2.5 mg/mL. The pka's of acyclovir are 2.27 and 9.25.
The Uses of Acyclovir sodium
Antiviral.
What are the applications of Application
Acyclovir sodium is a guanosine analog for biochemical research
Indications
Acyclovir sodium is used in certain people to treat outbreaks of herpes simplex infection of the skin (such as on the genitals), mucous membrane areas (such as the mouth and nose), or the brain. It is also used in certain people to treat shingles infection. The viruses that cause these infections live in the body quietly until an outbreak occurs. Acyclovir does not cure these infections but it can speed the healing of the sores, decrease pain/itching/formation of new sores, and lower the risk of other problems from the virus (such as infection spreading to other parts of the body/organs, pain even after the sores heal).
brand name
Zovirax (GlaxoSmithKline).
General Description
Acyclovir Sodium Injection is a synthetic nucleoside analogue active against herpes viruses. It is a sterile, aqueous solution for intravenous infusion, containing 50 mg acyclovir per mL in Water for Injection, USP. The concentration is equivalent to 54.9 mg of acyclovir sodium per mL in Water for Injection, USP. The sodium content is approximately 5.1 mg/mL. The pH range of the solution is 10.85 to 11.50. Further dilution of Acyclovir Sodium Injection in an appropriate intravenous solution must be performed before infusion.
Properties of Acyclovir sodium
| storage temp. | Keep in dark place,Inert atmosphere,Room temperature |
| InChI | InChI=1S/C8H11N5O3.Na.H/c9-8-11-6-5(7(15)12-8)10-3-13(6)4-16-2-1-14;;/h3,14H,1-2,4H2,(H3,9,11,12,15);; |
Safety information for Acyclovir sodium
| Signal word | Warning |
| Pictogram(s) |
 Exclamation Mark Irritant GHS07 |
| GHS Hazard Statements |
H302:Acute toxicity,oral |
| Precautionary Statement Codes |
P280:Wear protective gloves/protective clothing/eye protection/face protection. P305+P351+P338:IF IN EYES: Rinse cautiously with water for several minutes. Remove contact lenses, if present and easy to do. Continuerinsing. |
Computed Descriptors for Acyclovir sodium
| InChIKey | NHOSLZCNAFQLKZ-UHFFFAOYSA-N |
| SMILES | C(N1C=NC2C(NC(N)=NC1=2)=O)OCCO.[NaH] |
Acyclovir sodium manufacturer
New Products
Indole Methyl Resin tert-butyl 9-methoxy-3-azaspiro[5.5]undecane-3-carboxylate Boc-His(Boc)-OH 2-CTC Resin 4-Chloro-7-tosy1-7Hpyrrolo[2,3-d]pyrimidine 5,7-Dibromo-1H-indole 2,5-dichloro-N-hydroxy-4,6-dimethylpyridine-3-carboximidamide 2,2-Dimethoxy-7-azaspiro[3.5]nonane hydrochloride 4-chloromethyl-5-methyl-1,3-dioxol-2-one (DMDO-Cl) R-2-BENZYLOXY PROPIONIC ACID 1,1’-CARBONYLDIIMIDAZOLE 1,1’-CARBONYLDI (1,2-4 TRIAZOLE) N-METHYL INDAZOLE-3-CARBOXYLIC ACID 4-((2-hydroxyethyl)thio)benzoic acid 1-(TERT-BUTOXYCARBONYL)-2-PYRROLIDINONE Methyl 6-methylnicotinate 3-Pyridineacrylic acid tert-Butyl carbazate TETRAHYDRO-2H-PYRAN-3-OL 2-((4-morpholinophenylamino) (methylthio) methylene) malononitrile 3-(4-morpholinophenylamino)-5-amino-1H-pyrazole-4-carbonitrile 2,4-dihydroxybenzaldehyde 1,3-Diethyl-1,3-Diphenylurea Methyl 2-methylquinoline-6-carboxylateRelated products of tetrahydrofuran




You may like
-
 69657-51-8 Acyclovir sodium 98%View Details
69657-51-8 Acyclovir sodium 98%View Details
69657-51-8 -
 69657-51-8 98%View Details
69657-51-8 98%View Details
69657-51-8 -
 Acyclovir sodium 98%View Details
Acyclovir sodium 98%View Details
69657-51-8 -
 Acyclovir sodium 69657-51-8 98%View Details
Acyclovir sodium 69657-51-8 98%View Details
69657-51-8 -
 Acyclovir sodium 98%View Details
Acyclovir sodium 98%View Details
69657-51-8 -
 Aciclovir sodium 98% CAS 69657-51-8View Details
Aciclovir sodium 98% CAS 69657-51-8View Details
69657-51-8 -
 Sodium 2-((2-amino-6-oxo-1h-purin-9(6h)-yl)methoxy)ethanolate 98% CAS 69657-51-8View Details
Sodium 2-((2-amino-6-oxo-1h-purin-9(6h)-yl)methoxy)ethanolate 98% CAS 69657-51-8View Details
69657-51-8 -
 Thiourea 99% ARView Details
Thiourea 99% ARView Details
62-56-6
