Acetoin
Synonym(s):3-Hydroxy-2-butanone;Acetyl methyl carbinol, Acetoin;Acetylmethylcarbinol
- CAS NO.:513-86-0
- Empirical Formula: C4H8O2
- Molecular Weight: 88.11
- MDL number: MFCD00004521
- EINECS: 208-174-1
- SAFETY DATA SHEET (SDS)
- Update Date: 2025-12-17 09:49:33
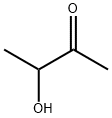
What is Acetoin?
Chemical properties
clear yellow solution
Chemical properties
Acetoin is a yellowish liquid with a bland, woody, yogurt odor and a fatty creamy “tub” butter taste. It is useful as a flavor ingredient in butter, milk, yogurt or strawberry flavors.
Occurrence
Reported found in fresh apple, butter, cheddar cheese, coffee, cocoa, honey, wheat bread and wine
The Uses of Acetoin
Acetoin is a produced via fermentation of wines, dairy products and sugars by fermentive bacteria. Acetoin is used in food flavoring and fragrances and is also found in some fruits and vegetables.
The Uses of Acetoin
Used as pharmaceutical intermediates, food spices; mainly for the preparation of cream, dairy, yogurt and strawberry spices.
The Uses of Acetoin
3-Hydroxy-2-butanone is a chemical used in food flavoring and fragrances. It acts as an intermediate of butanediol cycle in microorganisms. It is used as an aroma carrier in the preparation of flavors and essences.
What are the applications of Application
Acetoin is a chemical used in food flavoring and fragrances
Definition
ChEBI: A methyl ketone that is butan-2-one substituted by a hydroxy group at position 3.
Aroma threshold values
Aroma characteristics at 1.0%: strong buttery and creamy
Taste threshold values
Taste characteristics at 10 ppm: sweet, creamy, dairy, and butter-like.
General Description
A light-yellow colored liquid. Slightly denser than water. Hence sinks in water. Boiling point 280°F. Flash point between 100 and 141°F. Used to make other chemicals.
Air & Water Reactions
Flammable. Slightly soluble in water.
Reactivity Profile
3-Hydroxy-2-butanone is a ketone and alcohol. Ketones are reactive with many acids and bases liberating heat and flammable gases (e.g., H2). The amount of heat may be sufficient to start a fire in the unreacted portion of the ketone. Ketones react with reducing agents such as hydrides, alkali metals, and nitrides to produce flammable gas (H2) and heat. Ketones are incompatible with isocyanates, aldehydes, cyanides, peroxides, and anhydrides. They react violently with aldehydes, HNO3, HNO3 + H2O2, and HClO4. Flammable and/or toxic gases are generated by the combination of alcohols with alkali metals, nitrides, and strong reducing agents. They react with oxoacids and carboxylic acids to form esters plus water. Oxidizing agents convert them to aldehydes or ketones. Alcohols exhibit both weak acid and weak base behavior. They may initiate the polymerization of isocyanates and epoxides.
Health Hazard
Inhalation or contact with material may irritate or burn skin and eyes. Fire may produce irritating, corrosive and/or toxic gases. Vapors may cause dizziness or suffocation. Runoff from fire control may cause pollution.
Fire Hazard
HIGHLY FLAMMABLE: Will be easily ignited by heat, sparks or flames. Vapors may form explosive mixtures with air. Vapors may travel to source of ignition and flash back. Most vapors are heavier than air. They will spread along ground and collect in low or confined areas (sewers, basements, tanks). Vapor explosion hazard indoors, outdoors or in sewers. Runoff to sewer may create fire or explosion hazard. Containers may explode when heated. Many liquids are lighter than water.
Safety Profile
Experimental reproductive effects. LWdly toxic by subcutaneous route. A moderate skin irritant. Flammable liquid. When heated to decomposition it emits acrid smoke and fumes. See also KETONES
Synthesis
From diacetyl by partial reduction with zinc and acid. It is also a product of fermentation. Acetoin is an optically active compound. The d(–)acetyl methyl carbinol is obtained from fermentation and, in mixture with other products, from the catalytic oxidation of 2,3-butanediol. The 1(+)acetyl methyl carbinol is also obtained from fermentation. The optically pure form has not been isolated; the optically inactive form is prepared synthetically
Purification Methods
Wash acetoin with EtOH until colourless, then with diethyl ether or acetone to remove biacetyl. Dry it in air by suction and dry further in a vacuum desiccator. [Beilstein 1 IV 3991.]
Properties of Acetoin
| Melting point: | 15 °C (monomer) |
| Boiling point: | 148 °C(lit.) |
| Density | 1.013 g/mL at 25 °C(lit.) |
| vapor pressure | 86hPa at 20℃ |
| refractive index | n |
| FEMA | 2008 | ACETOIN |
| Flash point: | 123 °F |
| storage temp. | 2-8°C |
| solubility | H2O: 0.1 g/mL, clear |
| form | Liquid (Monomer) or Powder or Crystals (Dimer) |
| pka | 13.21±0.20(Predicted) |
| color | Pale yellow to green-yellow or white to yellow |
| Odor | buttery odor |
| Water Solubility | SOLUBLE |
| JECFA Number | 405 |
| Merck | 14,64 |
| BRN | 385636 |
| Stability: | Hygroscopic |
| CAS DataBase Reference | 513-86-0(CAS DataBase Reference) |
| NIST Chemistry Reference | 2-Butanone, 3-hydroxy-(513-86-0) |
| EPA Substance Registry System | 2-Butanone, 3-hydroxy- (513-86-0) |
Safety information for Acetoin
| Signal word | Danger |
| Pictogram(s) |
 Flame Flammables GHS02  Corrosion Corrosives GHS05 |
| GHS Hazard Statements |
H226:Flammable liquids H318:Serious eye damage/eye irritation |
| Precautionary Statement Codes |
P210:Keep away from heat/sparks/open flames/hot surfaces. — No smoking. P233:Keep container tightly closed. P240:Ground/bond container and receiving equipment. P241:Use explosion-proof electrical/ventilating/lighting/…/equipment. P280:Wear protective gloves/protective clothing/eye protection/face protection. P305+P351+P338:IF IN EYES: Rinse cautiously with water for several minutes. Remove contact lenses, if present and easy to do. Continuerinsing. |
Computed Descriptors for Acetoin
| InChIKey | ROWKJAVDOGWPAT-UHFFFAOYSA-N |
Acetoin manufacturer
Triveni Interchem Private Limited (Group Of Triveni Chemicals)
New Products
4,4-Difluoropiperidine hydrochloride tert-butyl 9-methoxy-3-azaspiro[5.5]undecane-3-carboxylate Indole Methyl Resin N-Isopropylurea N,N-Dicyclohexylcarbodiimide(DCC) MELDRUMS ACID 5-METHYLISOXAZOLE-4-CARBOXYLIC ACID Magnessium Bis glycinate Zinc ascorbate 1-bromo-2-butyne 2-acetamidophenol 9(10H)-anthracenone Erythrosin B, 4-Piperidinopiperidine 2-((4-morpholinophenylamino) (methylthio) methylene) malononitrile 2,4-dihydroxybenzaldehyde 3-(4-morpholinophenylamino)-5-amino-1H-pyrazole-4-carbonitrile Methyl 2-methylquinoline-6-carboxylate 2,6-dichloro-4-nitropyridine 4-Bromo-2-chlorobenzonitrile 2-(benzylamino)acetic acid hydrochloride 4-(tert-Butoxycarbonylamino)but- 2-ynoic acid 3,4-dihydro-2H-benzo[b][1,4]dioxepine 1-Phenyl-1-cycloprppanecarboxylicacidRelated products of tetrahydrofuran


You may like
-
![513-86-0 3-Hydroxybutan-2-one [Acetoin] 99%](https://img.chemicalbook.in//ProductImageIndia/2024-03/Raw/aaba6502-6f08-4a54-ba44-5a18d862172a.png) 513-86-0 3-Hydroxybutan-2-one [Acetoin] 99%View Details
513-86-0 3-Hydroxybutan-2-one [Acetoin] 99%View Details
513-86-0 -
 Acetyl methyl carbinol 99%View Details
Acetyl methyl carbinol 99%View Details
513-86-0 -
 Acetyl methyl carbinol 513-86-0 98%View Details
Acetyl methyl carbinol 513-86-0 98%View Details
513-86-0 -
 Acetoin 513-86-0 99%View Details
Acetoin 513-86-0 99%View Details
513-86-0 -
 Acetoin (May exist as crystalline dimer) CAS 513-86-0View Details
Acetoin (May exist as crystalline dimer) CAS 513-86-0View Details
513-86-0 -
 Acetoin CAS 513-86-0View Details
Acetoin CAS 513-86-0View Details
513-86-0 -
 Acetoin CAS 513-86-0View Details
Acetoin CAS 513-86-0View Details
513-86-0 -
 Hydrocarbon Solvents Acetoin, > 99%, IndustrialView Details
Hydrocarbon Solvents Acetoin, > 99%, IndustrialView Details
513-86-0
