Acetanilide
Synonym(s):N-Phenylacetamide;Acetanilide;N-Phenylacetamide
- CAS NO.:103-84-4
- Empirical Formula: C8H9NO
- Molecular Weight: 135.16
- MDL number: MFCD00008674
- EINECS: 203-150-7
- SAFETY DATA SHEET (SDS)
- Update Date: 2025-12-17 09:50:41
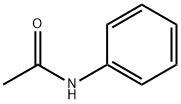
What is Acetanilide?
Chemical properties
Acetanilide, also known as Nphenylacetamide, acetanil, or acetanilide is a white to gray solid with molecular formula CH3CONHC6H5. It is an odorless colorless, glossy, crystalline powder or flakes. Acetanilide was the first aniline derivative found to possess analgesic as well as antipyretic properties and was quickly introduced into medical practice (Weast, 1981; Gnanasambandan et al., 2014). Later, it was established that in the human body it is mostly metabolized to paracetamol, this compound being responsible for the analgesic and antipyretic properties of acetanilide (Bertolini et al., 2006; Gnanasambandan et al., 2014). In addition, it was discovered that it has unacceptable toxic effects, so that acetanilide is no longer used as a drug.

Acetanilide is mainly used as an intermediates in the synthesis of pharmaceuticals and dyes, as an additive for hydrogen peroxide and cellulose ester varnishes, and as a plasticizer in polymer industry as well as accelerator in the rubber industry.
Physical properties
White glossy flake crystal or white crystalline powder. Slightly soluble in cold water, soluble in hot water, methanol, ethanol, ether, chloroform, acetone, glycerol and benzene.
History
The department of internal medicine at the University of Strassburg in the 1880s was noted for its investigations into intestinal worms. Adolf Kussmaul, the director, asked two young assistants, Arnold Cahn and Paul Hepp, to treat patients with naphthalene as it had been used elsewhere as an internal antiseptic. The young doctors were disappointed with the initial results, but Hepp persevered with the naphthalene treatment in a patient suffering from a variety of complaints besides worms. Surprisingly, the fever chart revealed a pronounced antipyretic effect from this treatment. This had not been observed before, but further investigation revealed that Hepp had wrongly been supplied by Kopp’s Pharmacy in Strassburg with acetanilide instead of naphthalene! Cahn and Hepp lost no time in publishing a report on their discovery of a new antipyretic.
For many years after its discovery in 1886, Acetanilide was used as an alternative to aspirin (i.e. acetyl salicylate) - an analgesic (painkiller) and antipyretic (fever reducing) drug to relieve e.g. headache, menstrual pain, and rheumatic pain. Under the name “Acetanilide” it formerly appeared in the formula of a number of patent medicines and over the counter drugs. In 1948, Julius Axelrod and Bernard Brodie discovered that Acetanilide is much more toxic in these applications than other drugs, causing methemoglobinemia and ultimately causing damage to the liver and kidneys. Thus, Acetanilide has largely been replaced by less toxic drugs, in particular acetaminophen (i.e. paracetamol), which is a metabolite of Acetanilide and whose use Axelrod and Brodie suggested in the same study.
The Uses of Acetanilide
Acetanilide is used as an intermediate in the synthesis of rubber accelerator, dyes and camphor. It is also used in the synthesis of penicillin and other pharmaceutical products. It is involved in the preparation of 4-acetamidobenzenesulfonyl chloride, which is an intermediate during the synthesis of sulfa drugs. Further, it is employed as a experimental photographic developer. In addition to this, it is used to stabilize cellulose ester varnishes.
What are the applications of Application
Acetanilide is useful for various organic synthesis reactions
Definition
ChEBI: Acetanilide is a member of the class of acetamides that is acetamide in which one of the hydrogens attached to the nitrogen is substituted by a phenyl group. It has a role as an analgesic. It is a member of acetamides and an anilide. It is functionally related to an acetic acid.
Preparation
Acetanilide is prepared from aniline by acetylating it with acetic anhydride in presence of glacial acetic acid. Aniline reacts with acetic anhydride to form Acetanilide by nucleophilic substitution reaction and the reaction is called acetylation.
In a 100 ml round bottom flask fitted with a reflux condensor place 5ml of aniline and 10 ml of 1:1 acetic acid and acetic anhydride mixture (5ml acetic acid and 5 ml acetic anhydride). Heat the mixture gently under reflux for 15-20 minutes on oil bath and then pour the contents while still hot with stirring into a 200ml beaker containing 100ml ice cold water. Stir the mixture vigorously to hydrolyse the excess acetic anhydride. After all the acetanilide has precipitated, collct it on buchner funnel and wash with cold water. Recrystallise the crude product from boiling water. If the product is excessively coloured add a pinch of animal charcoal to hot water and filter hot through glass wool/ cotton plug. Pure colourless crystals of acetanilide melts at 114°C (5-5.5g).
What are the applications of Application
Acetanilide is used as an inhibitor of hydrogen peroxide decomposition and to stabilize cellulose ester varnishes. It is also used in the intermediation of rubber accelerator synthesis, dyes and dye intermediate synthesis, and camphor synthesis. Acetanilide is used for the production of 4-acetamidobenzenesulfonyl chloride, a key intermediate for the manufacture of the sulfa drugs. It is also a precursor in the synthesis of penicillin and other pharmaceuticals. In the 19th century acetanilide was one of a large number of compounds used as experimental photographic developers.
Acetanilide is used as a EOF (electroosmotic flow) marker in the studies of affinity capillary electrophoresis for drug–protein binding.
Acetanilide undergoes palladium-catalyzed cross-coupling reaction to form ortho-acylacetanilide.
World Health Organization (WHO)
Acetanilide, a para-aminophenol derivative with analgesic, antipyretic and weak antiinflammatory activity, was introduced into medicine in 1886. It subsequently proved to be excessively myelosuppressive and has been superseded by safer alternatives.
Synthesis Reference(s)
Tetrahedron Letters, 24, p. 4533, 1983 DOI: 10.1016/S0040-4039(00)85947-X
General Description
Acetanilide is a white to gray solid. (NTP, 1992)
Air & Water Reactions
Acetanilide is sensitive to prolonged exposure to air . Water insoluble.
Reactivity Profile
Acetanilide is an amide. Flammable gases are formed by the reaction of organic amides with strong reducing agents. Amides are very weak bases (weaker than water). Imides are less basic yet and in fact react with strong bases to form salts. That is, they can react as acids. Mixing amides with dehydrating agents such as P2O5 or SOCl2 generates the corresponding nitrile. The combustion of these compounds generates mixed oxides of nitrogen (NOx).
Fire Hazard
Acetanilide is combustible.
Safety Profile
A human poison by an unspecified route. Poison by ingestion and intravenous routes. Moderately toxic by intraperitoneal route. Human systemic effects by ingestion: hallucinations and dstorted perceptions, sleepiness, constipation, cyanosis, respiratory stimulation, hdney damage, me themoglobinemiacarboxyhemoglobinemia, and decreased body temperature. Mutation data reported. When heated to decomposition it emits toxic fumes of NOx,. Combustible when exposed to heat or flame. See also ANILINE.
Potential Exposure
This amide compound is used in rubber industry as accelerator, in plastics industry as cellulose ester stabilizer, in pharmaceutical manufacture, stabilizer for hydrogen peroxide, azo dye manufacture
Shipping
UN2811 Toxic solids, organic, n.o.s., Hazard Class: 6.1; Labels: 6.1-Poisonous materials, Technical Name Required.
Purification Methods
Recrystallise acetanilide from water, aqueous EtOH, *benzene or toluene. [Beilstein 12 IV 373.]
Incompatibilities
Dust may form explosive mixture with air. Incompatible with oxidizers (chlorates, nitrates, peroxides, permanganates, perchlorates, chlorine, bromine, fluorine, etc.); contact may cause fires or explosions. Keep away from alkaline materials, strong bases, strong acids, oxoacids, epoxides, alkyl nitrates, alkalis (liberate aniline), chloral hydrate, phenols, ferric salts
Waste Disposal
Add to flammable solvents (alcohol or benzene) and incinerate. Oxides of nitrogenmay be scrubbed from combustion gases with alkaline solution
Properties of Acetanilide
| Melting point: | 113-115 °C(lit.) |
| Boiling point: | 304 °C(lit.) |
| Density | 1,121 g/cm3 |
| vapor density | 4.65 (vs air) |
| vapor pressure | 1 mm Hg ( 114 °C) |
| refractive index | 1.5700 (estimate) |
| Flash point: | 173 °C |
| storage temp. | Store below +30°C. |
| solubility | Slightly soluble in water; very soluble in ethanol and acetone; soluble in ethyl ether. |
| form | Powder |
| pka | 0.5(at 25℃) |
| color | Off-white to beige to grayish-blue |
| PH | 5-7 (10g/l, H2O, 25℃) |
| Water Solubility | 5 g/L (25 ºC) |
| Merck | 14,50 |
| BRN | 606468 |
| Dielectric constant | 2.9(22.0℃) |
| Stability: | Stable. Combustible. Incompatible with strong oxidizing agents, caustics, alkalies. |
| CAS DataBase Reference | 103-84-4(CAS DataBase Reference) |
| NIST Chemistry Reference | Acetamide, N-phenyl-(103-84-4) |
| EPA Substance Registry System | Acetanilide (103-84-4) |
Safety information for Acetanilide
| Signal word | Warning |
| Pictogram(s) |
 Exclamation Mark Irritant GHS07 |
| GHS Hazard Statements |
H302:Acute toxicity,oral |
Computed Descriptors for Acetanilide
Acetanilide manufacturer
Clickchem Research LLP
Luna Chemical Industries Private Limited
Zama Chemical
New Products
4,4-Difluoropiperidine hydrochloride tert-butyl 9-methoxy-3-azaspiro[5.5]undecane-3-carboxylate Indole Methyl Resin N-Isopropylurea N,N-Dicyclohexylcarbodiimide(DCC) MELDRUMS ACID 5-METHYLISOXAZOLE-4-CARBOXYLIC ACID Magnessium Bis glycinate Zinc ascorbate 1-bromo-2-butyne 2-acetamidophenol 9(10H)-anthracenone Erythrosin B, 4-Piperidinopiperidine 2-((4-morpholinophenylamino) (methylthio) methylene) malononitrile 2,4-dihydroxybenzaldehyde 3-(4-morpholinophenylamino)-5-amino-1H-pyrazole-4-carbonitrile Methyl 2-methylquinoline-6-carboxylate 2,6-dichloro-4-nitropyridine 4-Bromo-2-chlorobenzonitrile 2-(benzylamino)acetic acid hydrochloride 4-(tert-Butoxycarbonylamino)but- 2-ynoic acid 3,4-dihydro-2H-benzo[b][1,4]dioxepine 1-Phenyl-1-cycloprppanecarboxylicacidRelated products of tetrahydrofuran
![4-[1-(hydroxyamino)ethylidene]cyclohexa-2,5-dien-1-one](https://img.chemicalbook.in/CAS/GIF/34523-34-7.gif)


You may like
-
 Acetanilide (SQ) CAS 103-84-4View Details
Acetanilide (SQ) CAS 103-84-4View Details
103-84-4 -
 Acetanilide ExiPlus CAS 103-84-4View Details
Acetanilide ExiPlus CAS 103-84-4View Details
103-84-4 -
 Acetanilide extrapure CAS 103-84-4View Details
Acetanilide extrapure CAS 103-84-4View Details
103-84-4 -
 AcetanilideView Details
AcetanilideView Details
103-84-4 -
 Acetanilide flakes, For Industrial Usage, Grade: Technical GradeView Details
Acetanilide flakes, For Industrial Usage, Grade: Technical GradeView Details
103-84-4 -
 Acetanilide Chemical ., Grade Standard: BPView Details
Acetanilide Chemical ., Grade Standard: BPView Details
103-84-4 -
 Acetanilide Powder ChemicalView Details
Acetanilide Powder ChemicalView Details
103-84-4 -
 ACETANILIDE 98.5%View Details
ACETANILIDE 98.5%View Details
103-84-4
