Abacavir
- CAS NO.:136470-78-5
- Empirical Formula: C14H18N6O
- Molecular Weight: 286.33
- MDL number: MFCD00903850
- EINECS: 620-487-9
- SAFETY DATA SHEET (SDS)
- Update Date: 2024-12-18 14:15:30
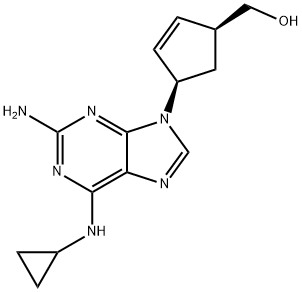
What is Abacavir?
Absorption
Rapid and extensive after oral administration (83% bioavailability, tablet). When a 300 mg tablet is given twice daily to subjects, the peak plasma concentration (Cmax) was 3.0 ± 0.89 mcg/mL and the area under the curve (AUC 0-12 hours) was 6.02 ± 1.73 mcg?hr/mL.
Toxicity
Some myocardial degeneration has been noticed in rats and mice. The most commonly reported adverse reactions of at least moderate intensity (incidence ≥10%) in adult HIV-1 clinical trials were nausea, headache, malaise and fatigue, nausea and vomiting, and dreams/sleep disorders. Serious hypersensitivity reactions have been associated with abacavir which has been strongly linked to the presence of the HLA-B*57:01 allele. This reaction manifests itself in patients within the first 6 weeks of treatment. Patients should be tested for the presence of this allele as recommended by the U.S Food and Drug Administration (FDA).
Description
The drug is extensively metabolized via stepwise phosphorylation to 5′-mono-, di-, and triphosphate. Abacavir is well absorbed (>75%) and penetrates the CNS. The drug can be taken without regard to meals. The drug does not show any clinically significant drug–drug interactions. Abacavir has been reported to produce life-threatening hypersensitivity reactions. The major use of abacavir appears to be in combination with other nucleoside RT inhibitors. A fixed-combination product has recently been approved by the U.S. FDA consisting of 300 mg of ABC, 150 mg of 3TC, and 300 mg of ZDV (Trizivar). The combination has been shown to be superior to other combinations in reducing viral load as well as to show improvement in CD4 cell count.
Description
Abacavir, is an antiretroviral drug. When a virus (such as HIV) tries to manufacture DNA from the viral RNA, it unknowingly incorporates abacavir instead of a natural component of DNA, guanosine, which stops the virus from reproducing. Suggested by Mamoun Abacavir.
Originator
Ziagen ,GlaxoSmithKline
The Uses of Abacavir
Abacavir is a commonly used nucleoside analogue with potent antiviral activity against HIV-1. - See more at: http://www.selleckchem.com/products/abacavir-sulfate.html#sthash.lApvcTNO.dpuf
The Uses of Abacavir
A nucleoside reverse transcriptase inhibitor (NRTI).
Background
Abacavir (ABC) is a powerful nucleoside analog reverse transcriptase inhibitor (NRTI) used to treat HIV and AIDS. Chemically, it is a synthetic carbocyclic nucleoside and is the enantiomer with 1S, 4R absolute configuration on the cyclopentene ring. In vivo, abacavir sulfate dissociates to its free base, abacavir.
What are the applications of Application
Ziagen is a carbocyclic nucleoside analogue with inhibitory activity against HIV-1
Indications
Abacavir is indicated in combination with other anti-retroviral agents for the treatment of HIV-1 infection. It is available in a combination product alongside dolutegravir and lamivudine for the treatment of adult and pediatric patients with HIV-1 who weigh ≥10 kg.
Indications
Abacavir (Ziagen) is a guanosine nucleoside analogue indicated for the therapy of HIV-1 infection in adults and children. It is used as part of a multidrug regimen and is available in a fixed-dose combination with zidovudine and lamivudine (Trizivir). It is also used for postexposure HIV infection prophylaxis.
Definition
ChEBI: Abacavir is a 2,6-diaminopurine that is (1S)-cyclopent-2-en-1-ylmethanol in which the pro-R hydrogen at the 4-position is substituted by a 2-amino-6-(cyclopropylamino)-9H-purin-9-yl group. A nucleoside analogue reverse transcriptase inhibitor (NRTI) with antiretroviral activity against HIV, it is used (particularly as the sulfate) with other antiretrovirals in combination therapy of HIV infection. It has a role as a HIV-1 reverse transcriptase inhibitor, an antiviral drug and a drug allergen.
Manufacturing Process
Treatment of 2,5-diamino-4,6-dihydroxypyrimidine (I) with
(chloromethylene)dimethylammonium chloride yielded the dichloropyrimidine
with both amino groups derivatized as amidines. Partial hydrolysis with
aqueous HCl in hot ethanol gave N-(2-amino-4,6-dichloro-pyrimidin-5-yl)-N,Ndimethylformamidene
(II). Subseqent buffered hydrolysis at pH 3.2 yielded
the (2-amino-4,6-dichloro-pyrimididin-5-ylamino)acetaldehyde (III).
Condensation chloropyrimidine (III) with (1S,4R)-4-amino-2-cyclopentene-1-
methanol (IV) in the presence of triethylamine and NaOH gave [2-amino-4-
chloro-6-(4-hydroxymethyl-cyclopent-2-enylamino)pyrimidin-5-ylamino]-
acetaldehyde (V). The correct enantiomer (IV) of racemic aminocyclopentene
was obtained by resolution of diastereomeric salts with D-dibenzoyltartaric
acid. Cyclization of (V) to the corresponding purine was accomplished with
refluxing triethyl orthoformate or diethoxymethyl acetate to give nucleoside
analogue [4-(2-amino-6-chloro-purin-9-yl)-cyclopent-2-enyl]methanol (VI).
Displacement of chloride in the purine nucleus with cyclopropyl amine in
refluxing butanol afforded abacavir. The structure of obtained compound was
confirmed by 1H NMR method and elemental analysis.
In practice it is usually used as sulfate salt.
Therapeutic Function
Antiviral
Antimicrobial activity
Abacavir has activity against HIV-1, HIV-2 and human T-cell lymphotrophic virus type-1 (HTLV-1).
Acquired resistance
Resistance is associated with specific changes in codons 184 with 65, 74 or 115 in the HIV reverse transcriptase codon region.
General Description
Abacavir is a nucleoside reverse transcriptase inhibitorNRTI that has been approved for use in combination therapiesfor the treatment of HIV and AIDS. Once in the tissues,it is metabolized by stepwise phosphorylation to themonophosphate, diphosphate, and triphosphate. Abacavir ishighly bioavailable (>75%) and is effective by the oralroute. It penetrates the blood-brain barrier efficiently.Abacavir has been reported to produce life-threatening hypersensitivityreactions in some patients.
Pharmaceutical Applications
A synthetic analog of guanine formulated for oral use.
Pharmacokinetics
Abacavir is a nucleoside reverse transcriptase inhibitor (NRTI) with activity against Human Immunodeficiency Virus Type 1 (HIV-1). Abacavir is phosphorylated to active metabolites that compete for incorporation into viral DNA. They inhibit the HIV reverse transcriptase enzyme competitively and act as a chain terminator of DNA synthesis. The concentration of drug necessary to effect viral replication by 50 percent (EC50) ranged from 3.7 to 5.8 μM (1 μM = 0.28 mcg/mL) and 0.07 to 1.0 μM against HIV-1IIIB and HIV-1BaL, respectively, and was 0.26 ± 0.18 μM against 8 clinical isolates. Abacavir had synergistic activity in cell culture in combination with the nucleoside reverse transcriptase inhibitor (NRTI) zidovudine, the non-nucleoside reverse transcriptase inhibitor (NNRTI) nevirapine, and the protease inhibitor (PI) amprenavir; and additive activity in combination with the NRTIs didanosine, emtricitabine, lamivudine, stavudine, tenofovir, and zalcitabine.
Pharmacokinetics
Oral absorption: 83%
Cmax 300 mg oral, twice daily: 3.0 ± 0.89 mg/L
600 mg once daily: 4.26 mg/L
Plasma half-life: 1.5 h
Volume of distribution: 0.8 L/kg
Plasma protein binding: c. 49%
Absorption
After oral administration abacavir sulfate undergoes rapid and extensive absorption unaffected by food.
Distribution
It penetrates well into the cerebrospinal fluid (CSF) and is an NRTI of choice if this characteristic is thought desirable. Good penetration into the male genital tract has been observed. The drug is secreted into human breast milk.
Metabolism
It is primarily metabolized in the liver, mainly by alcohol dehydrogenase and glucuronidation.
Excretion
Around 83% of the dose is eliminated in the urine, <2% as unchanged drug; the remainder is excreted in the feces. Dose adjustment is unnecessary in renal impairment. It can be used in moderate hepatic impairment, but is contraindicated if dysfunction is severe.
Clinical Use
Treatment of HIV infection in adults and children (in combination with other antiretroviral drugs)
Side Effects
Life-threatening hypersensitivity reactions occur in 5–8%
of all individuals, necessitating discontinuation of the drug.
Typically patients present within the first 6 weeks of starting
treatment with fever, rash or other symptoms that worsen
in severity with continued drug exposure. Hypersensitivity
is associated with carriage of the major histocompatibility
complex class I allele HLA-B57*01 and screening for this
allele can significantly reduce the incidence
of this effect.
Current or recent (within the preceding 6 months) use of
abacavir has been associated with a risk of myocardial infarction,
but studies have yielded conflicting data.
Side Effects
Abacavir is associated with side effects such as anorexia, nausea, vomiting, malaise, headache, and insomnia. A potentially fatal hypersensitivity reaction develops in approximately 5% of patients, usually early in the course of treatment. Fever and rash are the most common symptoms of this reaction; malaise, respiratory symptoms, and gastrointestinal complaints may also occur. Resistance to abacavir may be associated with resistance to zidovudine, didanosine, and lamivudine.
Drug interactions
Potentially hazardous interactions with other drugs
Antivirals: possibly reduces effects of ribavirin;
concentration reduced by tipranavir.
Orlistat: absorption possibly reduced by orlistat.
Metabolism
Hepatic, by alcohol dehydrogenase and glucuronosyltransferase to a 5′-carboxylic acid metabolite and 5′-glucuronide metabolite, respectively. These metabolites have no antiviral activity. Abacavir is not significantly metabolized by cytochrome P450 enzymes.
Metabolism
Abacavir is primarily metabolised by the liver with approximately 2% of the administered dose being renally excreted, as unchanged compound. The primary pathways of metabolism in man are by alcohol dehydrogenase and by glucuronidation to produce the 5'-carboxylic acid and 5'-glucuronide which account for about 66% of the administered dose. The metabolites are excreted in the urine.
Properties of Abacavir
| Melting point: | 165° |
| Boiling point: | 636.0±65.0 °C(Predicted) |
| alpha | D20 -59.7°; 43620 -127.8°; 36520 -218.1° (c = 0.15 in methanol) |
| Density | 1.70±0.1 g/cm3(Predicted) |
| storage temp. | Sealed in dry,2-8°C |
| solubility | DMSO (Slightly, Heated), Methanol (Slightly) |
| pka | 5.01(at 25℃) |
| form | Solid |
| color | Off-White to Pale Beige |
| Merck | 14,1 |
| CAS DataBase Reference | 136470-78-5(CAS DataBase Reference) |
| EPA Substance Registry System | 2-Cyclopentene-1-methanol, 4-[2-amino-6-(cyclopropylamino)-9H-purin-9-yl]-, (1S,4R)- (136470-78-5) |
Safety information for Abacavir
| Signal word | Danger |
| Pictogram(s) |
 Health Hazard GHS08 |
| GHS Hazard Statements |
H317:Sensitisation, Skin H334:Sensitisation, respiratory H341:Germ cell mutagenicity H361:Reproductive toxicity |
| Precautionary Statement Codes |
P201:Obtain special instructions before use. P202:Do not handle until all safety precautions have been read and understood. P261:Avoid breathing dust/fume/gas/mist/vapours/spray. P272:Contaminated work clothing should not be allowed out of the workplace. P280:Wear protective gloves/protective clothing/eye protection/face protection. P284:Wear respiratory protection. P302+P352:IF ON SKIN: wash with plenty of soap and water. P304+P340:IF INHALED: Remove victim to fresh air and Keep at rest in a position comfortable for breathing. P308+P313:IF exposed or concerned: Get medical advice/attention. P333+P313:IF SKIN irritation or rash occurs: Get medical advice/attention. P405:Store locked up. |
Computed Descriptors for Abacavir
Abacavir manufacturer
HRV Global Life Sciences
Arene Lifesciences Limited
Vishrudh laboratories pvt ltd
Vijaya Pharma And Life Science
KPS Chemicals And Pharmaceuticals
New Products
Tert-butyl bis(2-chloroethyl)carbamate 4-Methylphenylacetic acid N-Boc-D-alaninol N-BOC-D/L-ALANINOL N-octanoyl benzotriazole 3-Morpholino-1-(4-nitrophenyl)-5,6-dihydropyridin- 2(1H)-one Furan-2,5-Dicarboxylic Acid DIETHYL AMINOMALONATE HYDROCHLORIDE 1,1’-CARBONYLDIIMIDAZOLE R-2-BENZYLOXY PROPIONIC ACID 1,1’-CARBONYLDI (1,2-4 TRIAZOLE) N-METHYL INDAZOLE-3-CARBOXYLIC ACID (2-Hydroxyphenyl)acetonitrile 4-Bromopyrazole 5-BROMO-2CYANO PYRIDINE 5,6-Dimethoxyindanone 5-broMo-2-chloro-N-cyclopentylpyriMidin-4-aMine 2-(Cyanocyclohexyl)acetic acid 4-methoxy-3,5-dinitropyridine 1-(4-(aminomethyl)benzyl)urea hydrochloride 2-aminopropyl benzoate hydrochloride diethyl 2-(2-((tertbutoxycarbonyl)amino) ethyl)malonate tert-butyl 4- (ureidomethyl)benzylcarbamate Ethyl-2-chloro((4-methoxyphenyl)hydrazono)acetateRelated products of tetrahydrofuran




You may like
-
 Abacavir 98%View Details
Abacavir 98%View Details
136470-78-5 -
 Abacavir 136470-78-5 99%View Details
Abacavir 136470-78-5 99%View Details
136470-78-5 -
 Abacavir CAS 136470-78-5View Details
Abacavir CAS 136470-78-5View Details
136470-78-5 -
 136470-78-5 98%View Details
136470-78-5 98%View Details
136470-78-5 -
 136470-78-5 98%View Details
136470-78-5 98%View Details
136470-78-5 -
 Abacavir 98%View Details
Abacavir 98%View Details
136470-78-5 -
 Abacavir >99% (HPLC) CAS 136470-78-5View Details
Abacavir >99% (HPLC) CAS 136470-78-5View Details
136470-78-5 -
 Abacavir 95.00% CAS 136470-78-5View Details
Abacavir 95.00% CAS 136470-78-5View Details
136470-78-5