6-Maleimidocaproic acid
Synonym(s):MCA;6-Maleimidocaproic acid;Maleimidocaproic acid;N-Maleoyl-6-aminocaproic acid;2,5-Dihydro-2,5-dioxo-1H-pyrrole-1-hexanoic acid
- CAS NO.:55750-53-3
- Empirical Formula: C10H13NO4
- Molecular Weight: 211.21
- MDL number: MFCD00043140
- EINECS: 611-311-1
- SAFETY DATA SHEET (SDS)
- Update Date: 2024-12-18 14:15:30
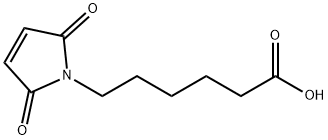
What is 6-Maleimidocaproic acid?
Description
6-Maleimidocaproic acid contains a maleimide group and a terminal carboxylic acid. The terminal carboxylic acid can react with primary amine groups in the presence of activators (e.g. EDC, or HATU) to form a stable amide bond. The maleimide group will react with a thiol group to form a covalent bond, enabling the connection of biomolecule with a thiol.
Chemical properties
White to yellow solid, powder, crystals or crystaline powder.
The Uses of 6-Maleimidocaproic acid
6-Maleimidohexanoic acid is used as a probe for introducing maleimides groups into biomolecules and active pharmaceutical ingredients. Further, it is used with N-hydroxysuccinimide ester as a bifunctional cross-linking reagent. In addition to this, it acts as a probe for thiol groups in membrane proteins.
Definition
ChEBI: 6-Maleimidocaproic acid is a medium-chain fatty acid. It contains a maleimide group and a terminal carboxylic acid. The terminal carboxylic acid can react with primary amine groups in the presence of activators (e.g. EDC, or HATU) to form a stable amide bond. The maleimide group will react with a thiol group to form a covalent bond, enabling the connection of biomolecule with a thiol.
What are the applications of Application
6-Maleimidocaproic acid is a sulfhydryl reactive heterobifunctional crosslinking reagent. It can be widely used probe for introducing maleimides groups into biomolecules. A probe for thiol groups in proteins. Spacer Arm: 9.4 Angstroms. Probe for thiol groups (SH-groups) in membrane proteins.
Synthesis
Maleic anhydride (29.4 g, 0.3 mol) and 6-aminocaproic acid (39.35 g, 0.3 mol) were refluxed in
glacial acetic acid (900 mL) for 16 h. Acetic anhydride (30.6 g, 0.3 mol) was added dropwise over
a period of 2 hand reflux was continued for 1 h. The acetic acid was removed under vacuum at 70
°C to yield yellow syrup which solidified. The material was chromatographed over silica using
dichloromethane-methanol-acetic acid (100:5:1) affording a crystalline solid (6-Maleimidocaproic acid).
Properties of 6-Maleimidocaproic acid
| Melting point: | 86-91 °C |
| Boiling point: | 407.3±28.0 °C(Predicted) |
| Density | 1.285±0.06 g/cm3(Predicted) |
| storage temp. | 2-8°C |
| solubility | DMSO (Slightly), Ethyl Acetate (Slightly) |
| pka | 4.74±0.10(Predicted) |
| form | Powder |
| color | White to pale yellow |
| Water Solubility | Soluble in water, methanol, ethanol, dimethyl sulfoxide. Insoluble in ether. |
| Sensitive | Moisture Sensitive |
| BRN | 1532405 |
| CAS DataBase Reference | 55750-53-3(CAS DataBase Reference) |
| EPA Substance Registry System | 1H-Pyrrole-1-hexanoic acid, 2,5-dihydro-2,5-dioxo- (55750-53-3) |
Safety information for 6-Maleimidocaproic acid
| Signal word | Danger |
| Pictogram(s) |
 Corrosion Corrosives GHS05  Exclamation Mark Irritant GHS07 |
| GHS Hazard Statements |
H315:Skin corrosion/irritation H318:Serious eye damage/eye irritation H335:Specific target organ toxicity, single exposure;Respiratory tract irritation |
| Precautionary Statement Codes |
P280:Wear protective gloves/protective clothing/eye protection/face protection. P302+P352:IF ON SKIN: wash with plenty of soap and water. |
Computed Descriptors for 6-Maleimidocaproic acid
| InChIKey | WOJKKJKETHYEAC-UHFFFAOYSA-N |
New Products
4-Fluorophenylacetic acid 4-Methylphenylacetic acid N-Boc-D-alaninol N-BOC-D/L-ALANINOL Tert-butyl bis(2-chloroethyl)carbamate 3-Morpholino-1-(4-nitrophenyl)-5,6-dihydropyridin- 2(1H)-one Furan-2,5-Dicarboxylic Acid Tropic acid S-2-CHLORO PROPIONIC ACID ETHYL ISOCYANOACETATE 2-Bromo-1,3-Bis(Dimethylamino)Trimethinium Hexafluorophosphate (6-METHYL-[1,3]DITHIOLO[4,5-b]QUINOXALIN-2-ONE INDAZOLE-3-CARBOXYLIC ACID 4-IODO BENZOIC ACID (2-Hydroxyphenyl)acetonitrile 4-Bromopyrazole 5,6-Dimethoxyindanone 2-(Cyanocyclohexyl)acetic acid 4-methoxy-3,5-dinitropyridine 2-aminopropyl benzoate hydrochloride 1-(4-(aminomethyl)benzyl)urea hydrochloride diethyl 2-(2-((tertbutoxycarbonyl)amino) ethyl)malonate tert-butyl 4- (ureidomethyl)benzylcarbamate Ethyl-2-chloro((4-methoxyphenyl)hydrazono)acetateRelated products of tetrahydrofuran






You may like
-
 6-Maleimidohexanoic acid CAS 55750-53-3View Details
6-Maleimidohexanoic acid CAS 55750-53-3View Details
55750-53-3 -
 6-Maleimidohexanoic Acid CAS 55750-53-3View Details
6-Maleimidohexanoic Acid CAS 55750-53-3View Details
55750-53-3 -
 6-Maleimidohexanoic acid CAS 55750-53-3View Details
6-Maleimidohexanoic acid CAS 55750-53-3View Details
55750-53-3 -
 6-Maleimidohexanoic acid CAS 55750-53-3View Details
6-Maleimidohexanoic acid CAS 55750-53-3View Details
55750-53-3 -
 1975-50-4 98%View Details
1975-50-4 98%View Details
1975-50-4 -
 2-HYDROXY BENZYL ALCOHOL 98%View Details
2-HYDROXY BENZYL ALCOHOL 98%View Details
90-01-7 -
 14714-50-2 (2-Hydroxyphenyl)acetonitrile 98+View Details
14714-50-2 (2-Hydroxyphenyl)acetonitrile 98+View Details
14714-50-2 -
 118753-70-1 98+View Details
118753-70-1 98+View Details
118753-70-1