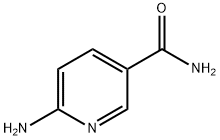
6-Aminopyridine-3-carboxamide
Synonym(s):6-Aminopyridine-3-carboxamide;6AN
- CAS NO.:329-89-5
- Empirical Formula: C6H7N3O
- Molecular Weight: 137.14
- MDL number: MFCD00006327
- EINECS: 206-349-7
- SAFETY DATA SHEET (SDS)
- Update Date: 2024-11-19 15:53:33
What is 6-Aminopyridine-3-carboxamide?
The Uses of 6-Aminopyridine-3-carboxamide
antineoplastic, apoptosis inducer
The Uses of 6-Aminopyridine-3-carboxamide
6-Aminonicotinamide induces apoptosis in tumor cells. It is clinically used in disseminated neoplastic disease. It also acts as 6-phosphogluconate dehydrogenase inhibitor. It aids in the treatment of psoriasis. It is used as cancer chemotherapeutic drug in animals.
What are the applications of Application
6-Aminonicotinamide is a PGD inhibitor and apoptosis inducer
Definition
ChEBI: A monocarboxylic acid amide resulting from the formal condensation of the carboxy group of 6-aminonicotinic acid with ammonia. An inhibitor of the NADP+-dependent enzyme, 6-phosphogluconate dehydrogenase, it interferes with glycolysis resulting in ATP depletion and synergizes with DNA-crosslinking chemotherapy drugs, such as cisplatin, in killing cancer cells.
General Description
White crystalline solid.
Air & Water Reactions
Insoluble in water.
Reactivity Profile
An amine and amide. Amines are chemical bases. They neutralize acids to form salts plus water. These acid-base reactions are exothermic. The amount of heat that is evolved per mole of amine in a neutralization is largely independent of the strength of the amine as a base. Amines may be incompatible with isocyanates, halogenated organics, peroxides, phenols (acidic), epoxides, anhydrides, and acid halides. Flammable gaseous hydrogen is generated by amines in combination with strong reducing agents, such as hydrides. Organic amides/imides react with azo and diazo compounds to generate toxic gases. Flammable gases are formed by the reaction of organic amides/imides with strong reducing agents. Amides are very weak bases (weaker than water). Imides are less basic yet and in fact react with strong bases to form salts. That is, they can react as acids. Mixing amides with dehydrating agents such as P2O5 or SOCl2 generates the corresponding nitrile. The combustion of these compounds generates mixed oxides of nitrogen (NOx).
Health Hazard
ACUTE/CHRONIC HAZARDS: When heated to decomposition 6-Aminopyridine-3-carboxamide emits toxic fumes of NOx.
Fire Hazard
Flash point data for 6-Aminopyridine-3-carboxamide are not available; however, 6-Aminopyridine-3-carboxamide is probably combustible.
Biological Activity
ki: 0.46 μm6-aminonicotinamide is a 6-phosphogluconate dehydrogenase inhibitor.6-phosphogluconate dehydrogenase, an enzyme in the pentose phosphate pathway, can produce ribulose 5-phosphate from 6-phosphogluconate. 6-phosphogluconate dehydrogenase is also an oxidative carboxylase catalyzing the decarboxylating reduction of 6-phosphogluconate into ribulose 5-phosphate in the presence of nadp.
in vitro
6-aminonicotinamide could be metabolized to 6-amino-nad(p+), a competitive inhibitor of nad(p+)-requiring processes, especially the pentose phosphate pathway enzyme, 6-phosphogluconate dehydrogenase. moreove, 6-aminonicotinamide as a single agent could cause a significant inhibition of glycolytic flux but had no effect on the pentose phosphate pathway. 31p-nmr studies of perifused rif-1 cells indicated that 4 h of exposure to 6-aminonicotinamide was enough to cause significant accumulation of 6-phosphogluconate, the substrate for this enzyme [1].
in vivo
the influence of 6-aminonicotinamide on the g-6-p-dh- and 6-pg-dh-levels of the pentose phosphate pathway in the kidney was investigated. following i.p. administration of 6-aminonicotinamide at 6 mg/kg, the 6-pg-level rose from a value less than 10 nmoles/g to 1000 nmoles/g fresh weight within 24 h. in addition, at the end of the assay, after 7 days, a 6-pg-concentration of about 150 nmoles/g fresh weight was still seen. moreove, the g-6-p level simultaneously rose with the 6-pg-level to about 150% of the control level and returned to normal on the 5th day [2].
References
[1] street, j. c.,alfieri, a.a. and koutcher, j.a. quantitation of metabolic and radiobiological effects of 6-aminonicotinamide in rif-1 tumor cells in vitro. cancer research 57, 3956-3962 (1997).
[2] lange, k. and proft, e.r. inhibition of the 6-phosphogluconate dehydrogenase in the rat kidney by 6-aminonicotinamide. naunyn-schmiedeberg's archives of pharmacology 267, 177-180 (1970).
Properties of 6-Aminopyridine-3-carboxamide
| Melting point: | 245-248 °C (lit.) |
| Boiling point: | 251.97°C (rough estimate) |
| Density | 1.2620 (rough estimate) |
| refractive index | 1.6910 (estimate) |
| storage temp. | Keep in dark place,Inert atmosphere,Room temperature |
| solubility | insoluble in H2O; insoluble in EtOH; ≥13.7 mg/mL in DMSO |
| pka | 15.66±0.50(Predicted) |
| form | powder to crystal |
| color | White to Light yellow |
| Water Solubility | Soluble in Dimethyl sulfoxide (DMSO). Insoluble in water. |
| BRN | 116042 |
| CAS DataBase Reference | 329-89-5(CAS DataBase Reference) |
| NIST Chemistry Reference | Nicotinamide, 6-amino-(329-89-5) |
| EPA Substance Registry System | 6-Aminonicotinamide (329-89-5) |
Safety information for 6-Aminopyridine-3-carboxamide
| Signal word | Danger |
| Pictogram(s) |
 Health Hazard GHS08 |
| Precautionary Statement Codes |
P201:Obtain special instructions before use. P308+P313:IF exposed or concerned: Get medical advice/attention. |
Computed Descriptors for 6-Aminopyridine-3-carboxamide
New Products
4-AMINO-TETRAHYDRO-PYRAN-4-CARBOXYLIC ACID HCL 4-(Dimethylamino)tetrahydro-2H-pyran-4-carbonitrile 4-Aminotetrahydropyran-4-carbonitrile Hydrochloride (R)-3-Aminobutanenitrile Hydrochloride 3-((Dimethylamino)methyl)-5-methylhexan-2-one oxalate 1,4-Dioxa-8-azaspiro[4.5]decane 5-Bromo-2-nitropyridine Nimesulide BP Aceclofenac IP/BP/EP Diclofenac Sodium IP/BP/EP/USP Mefenamic Acid IP/BP/EP/USP Ornidazole IP Diclofenac Potassium THOMAIND PAPER PH 2.0 TO 4.5 1 BOX BUFFER CAPSULE PH 9.2 - 10 CAP SODIUM CHLORIDE 0.1N CVS ALLOXAN MONOHYDRATE 98% PLATINUM 0.5% ON 3 MM ALUMINA PELLETS (TYPE 73) LITHIUM AAS SOLUTION 2-Bromo-1-(bromomethyl)-3-chloro-5-nitrobenzene 2-Bromo-3-nitroaniline N-(3-Hydroxypropyl)-N-methylacetamide 3-Bromo-6-chloropyridazine 4-ethyl-3-nitrobenzoic acidRelated products of tetrahydrofuran



You may like
-
 6-Aminonicotinamide CAS 329-89-5View Details
6-Aminonicotinamide CAS 329-89-5View Details
329-89-5 -
 6-Aminonicotinamide, 99% CAS 329-89-5View Details
6-Aminonicotinamide, 99% CAS 329-89-5View Details
329-89-5 -
 6-Aminonicotinamide CAS 329-89-5View Details
6-Aminonicotinamide CAS 329-89-5View Details
329-89-5 -
 1823368-42-8 98%View Details
1823368-42-8 98%View Details
1823368-42-8 -
 2-(3-(tert-butyl)phenoxy)-2-methylpropanoic acid 1307449-08-6 98%View Details
2-(3-(tert-butyl)phenoxy)-2-methylpropanoic acid 1307449-08-6 98%View Details
1307449-08-6 -
 Ethyl 3-(furan-2-yl)-3-hydroxypropanoate 25408-95-1 98%View Details
Ethyl 3-(furan-2-yl)-3-hydroxypropanoate 25408-95-1 98%View Details
25408-95-1 -
 2-Chloro-5-fluoro-1-methoxy-3-methylbenzene 98%View Details
2-Chloro-5-fluoro-1-methoxy-3-methylbenzene 98%View Details
1805639-70-6 -
 Lithium ClavulanateView Details
Lithium ClavulanateView Details
61177-44-4