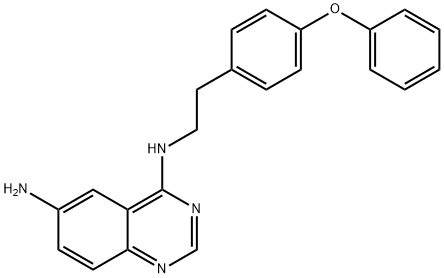
6-AMINO-4-(4-PHENOXYPHENYLETHYLAMINO)QUINAZOLINE
Synonym(s):4-N-[2-(4-Phenoxyphenyl)ethyl]quinazoline-4,6-diamine;6-Amino-4-(4-phenoxyphenylethylamino)quinazoline;N4-[2-(4-Phenoxyphenyl)ethyl]-4,6-quinazolinediamine;NF-κB Activation Inhibitor - CAS 545380-34-5 - Calbiochem;QNZ
- CAS NO.:545380-34-5
- Empirical Formula: C22H20N4O
- Molecular Weight: 356.42
- MDL number: MFCD06411436
- SAFETY DATA SHEET (SDS)
- Update Date: 2024-11-19 23:02:33
What is 6-AMINO-4-(4-PHENOXYPHENYLETHYLAMINO)QUINAZOLINE?
Description
QNZ (545380-34-5) was originally described as a potent inhibitor of NF-κB activation (IC50?= 11 nM) and TNF-α?production (IC50?= 7 nM).1,2?It indirectly inhibits the NF-κB pathway via inhibition of store-operated calcium entry (SOC) and displayed neuroprotective effects in transgenic fly and mouse models of Huntington’s disease.3,4?Its target has been postulated to be heteromeric calcium channels containing TRPC1 as one of the subunits.4?QNZ reduced synaptic neuronal SOC and rescued dendritic spine loss in YAC128 striatal medium spiny neurons.5?QNZ has also been identified as a potent (IC50?= 25 nM complex 1 from?Y.lipolytica; IC50?= 14 nM complex 1 from?Bos Taurus?heart mitochondria) and selective inhibitor of mitochondrial complex I.6?QNZ?decreased PSEN1ΔE9-mediated nSOCE upregulation and rescued mushroom spines in PSEN1ΔE9-expressing neurons, which are linked to familial Alzheimer’s disease.7
Chemical properties
White Solid
The Uses of 6-AMINO-4-(4-PHENOXYPHENYLETHYLAMINO)QUINAZOLINE
QNZ is a novel inhibitor of NFkB which displays potent inhibitory effects on both NFKB transcriptional activation (IC50=11nM) and TNF-a production (IC50=7nM). It dose-dependently inhibited carragenin-induced edema formation in the rat paw model (1m
The Uses of 6-AMINO-4-(4-PHENOXYPHENYLETHYLAMINO)QUINAZOLINE
QNZ is an inhibitor of NF-κB activation with IC50 of 11 nM.
What are the applications of Application
QNZ is an inhibitor of the transcription factor NFκB
References
1) Tobe?et al. (2003),?Discovery of quinazolines as a novel structural class of potent inhibitors of NF-kappa B activation; Bioorg. Med. Chem. Lett,?11?383 2) Tobe?et al.?(2003),?A novel structural class of potent inhibitors of NF-kB activation: structure-activity relationships and biological effects of 6-aminoquinazoline derivatives; Bioorg. Med. Chem. Lett,?11?3869 3) Choi?et al.?(2006),?Nuclear factor-kappaB activated by capacitive Ca2+ entry enhances muscarinic receptor-mediated soluble amyloid precursor protein (sAPPalpha) release in SH-SY5Y cells; J. Biol. Chem.,?281?12722 4) Wu?et al.?(2011),?Neuronal Store-Operated Calcium Entry Pathway as a Novel Therapeutic Target for Huntington’s Disease Treatment; Chem. Biol.,?18?777 5) Wu?et al. (2016),?Enhanced Store-Operated Calcium Entry Leads to Striatal Synaptic Loss in a Huntington’s Disease Mouse Model; J. Neurosci.,?36?125 6) Krishnathas?et al. (2017),?Identification of 4-N-[2-(4-phenoxyphenyl)ethyl]quinazoline-4,6-diamine as a novel, highly potent and specific inhibitor of mitochondrial complex I; Medchemcomm.?8?657 7) Chernyuk?et al. (2019),?Antagonist of neuronal store-operated calcium entry exerts beneficial effects in neurons expressing PSEN1?E9 mutant linked to familial Alzheimer disease;?Neuroscience,?410?118
Properties of 6-AMINO-4-(4-PHENOXYPHENYLETHYLAMINO)QUINAZOLINE
| Melting point: | 169-175°C |
| storage temp. | Keep in dark place,Inert atmosphere,2-8°C |
| solubility | DMSO: soluble15mg/mL, clear |
| form | powder |
| color | white to beige |
| Stability: | Stable for 1 year from date of purchase as supplied. Solutions in DMSO may be stored at -20° for up to 1 month. |
Safety information for 6-AMINO-4-(4-PHENOXYPHENYLETHYLAMINO)QUINAZOLINE
Computed Descriptors for 6-AMINO-4-(4-PHENOXYPHENYLETHYLAMINO)QUINAZOLINE
New Products
(S)-3-Aminobutanenitrile hydrochloride 4-Methylphenylacetic acid N-Boc-D-alaninol N-BOC-D/L-ALANINOL Tert-butyl bis(2-chloroethyl)carbamate 3-Morpholino-1-(4-nitrophenyl)-5,6-dihydropyridin- 2(1H)-one Furan-2,5-Dicarboxylic Acid Tropic acid 1-Bromo-3,5-Di-Tert-Butylbenzene S-2-CHLORO PROPIONIC ACID ETHYL ISOCYANOACETATE 2-Bromo-1,3-Bis(Dimethylamino)Trimethinium Hexafluorophosphate 4-IODO BENZOIC ACID 3-NITRO-2-METHYL ANILINE 1-(2,4-DICHLOROPHENYL) ETHANAMINE (2-Hydroxyphenyl)acetonitrile 4-Bromopyrazole 2-(Cyanocyclohexyl)acetic acid 4-methoxy-3,5-dinitropyridine 1-(4-(aminomethyl)benzyl)urea hydrochloride 2-aminopropyl benzoate hydrochloride diethyl 2-(2-((tertbutoxycarbonyl)amino) ethyl)malonate tert-butyl 4- (ureidomethyl)benzylcarbamate Ethyl-2-chloro((4-methoxyphenyl)hydrazono)acetateRelated products of tetrahydrofuran



You may like
-
 NF-κB Activation Inhibitor CASView Details
NF-κB Activation Inhibitor CASView Details -
 NF-κB Activation Inhibitor CAS 545380-34-5View Details
NF-κB Activation Inhibitor CAS 545380-34-5View Details
545380-34-5 -
 1975-50-4 98%View Details
1975-50-4 98%View Details
1975-50-4 -
 2-HYDROXY BENZYL ALCOHOL 98%View Details
2-HYDROXY BENZYL ALCOHOL 98%View Details
90-01-7 -
 2-Chloro-1,3-Bis(Dimethylamino)Trimethinium Hexafluorophosphate 221615-75-4 98%View Details
2-Chloro-1,3-Bis(Dimethylamino)Trimethinium Hexafluorophosphate 221615-75-4 98%View Details
221615-75-4 -
 61397-56-6 CIS BROMO BENZOATE 98%View Details
61397-56-6 CIS BROMO BENZOATE 98%View Details
61397-56-6 -
 14714-50-2 (2-Hydroxyphenyl)acetonitrile 98+View Details
14714-50-2 (2-Hydroxyphenyl)acetonitrile 98+View Details
14714-50-2 -
 118753-70-1 98+View Details
118753-70-1 98+View Details
118753-70-1