5-(TETRADECYLOXY)-2-FUROIC ACID
Synonym(s):5-(Tetradecyloxy)-2-furoic acid;TOFA - CAS 54857-86-2 - Calbiochem
- CAS NO.:54857-86-2
- Empirical Formula: C19H32O4
- Molecular Weight: 324.45
- MDL number: MFCD01726059
- SAFETY DATA SHEET (SDS)
- Update Date: 2024-11-15 19:19:13
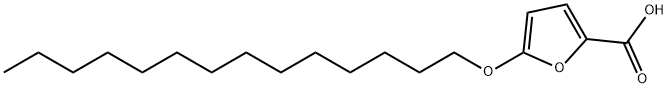
What is 5-(TETRADECYLOXY)-2-FUROIC ACID?
Description
TOFA (54857-86-2) interferes with fatty acid synthase via inhibition of acetyl Co-A carboxylase (ACC1).1 Induces apoptosis in a variety of tumor cell lines.2,3 Stimulates neurite outgrowth and neuronal differentiation in rat pheochromocytoma cells.4 TOFA impairs glucose-stimulated insulin secretion after chronic treatment.5
The Uses of 5-(TETRADECYLOXY)-2-FUROIC ACID
TOFA has been used as a lipid biosynthesis inhibitor in mesenchymal stromal cells (MSCs), human pluripotent stem cells., an acetyl-CoA carboxylase 1 inhibitor in murine adipocyte cell lines., a lipolysis inhibitor in cancer stem cells (CSCs).
What are the applications of Application
TOFA (5-(Tetradecyloxy) -2-furoic acid) is an inhibitor of ACC and fatty acid synthesis
Definition
ChEBI: A member of the class of furans that is 2-furoic acid in which the hydrogen at position 5 is replaced by a tetradecyloxy group.
Biochem/physiol Actions
5-(Tetradecyloxy)-2-furoic acid (TOFA) elicits hypolipidemic?functionality by favoring fatty acid breakdown and at the same time preventing biosynthesis. It induces apoptosis in pancreatic cancer cells and favors tumor suppression.
References
References/Citations: 1) Halvorson?et al. (1984),?Inhibition of fatty acid synthesis in isolated adipocytes by 5-tetradecyloxy)-2-furoic acid; Lipids,?19?851 2) Guseva?et al. (2011),?TOFA (5-tetradecyl-oxy-2-furoic acid) reduces fatty acid synthesis, inhibits expression of AR, neuropilin-1 and Mcl-1 and kills prostate cancer cells independent of p53 status; Cancer Biol. Ther.,?12?80 3) Zhou?et al. (2003),?Fatty acid synthesis inhibition triggers apoptosis during S phase in human cancer cells; Cancer Res.,?63?7330 4) Schmidt?et al. (1999),?Transcription control and neuronal differentiation by agents that activate the LXR nuclear receptor family; Mol. Cell. Endocrinol.,?155?51 5) Ronnebaum?et al. (2008),?Chronic suppression of acetyl-CoA carboxylase 1 in beta-cells impairs insulin secretion via inhibition of glucose rather than lipid metabolism; J. Biol. Chem.,?283?14248
Properties of 5-(TETRADECYLOXY)-2-FUROIC ACID
| Melting point: | 112-115 °C |
| Boiling point: | 441.7±25.0 °C(Predicted) |
| Density | 1.003±0.06 g/cm3(Predicted) |
| storage temp. | -20°C |
| solubility | DMSO: 2.5 mg/mL |
| form | solid |
| pka | 3.55±0.10(Predicted) |
| color | white to beige |
| Stability: | Stable for 1 year from date of purchase as supplied. Solutions in DMSO or ethanol may be stored at -20° for up to 1 month. |
Safety information for 5-(TETRADECYLOXY)-2-FUROIC ACID
Computed Descriptors for 5-(TETRADECYLOXY)-2-FUROIC ACID
New Products
4-AMINO-TETRAHYDRO-PYRAN-4-CARBOXYLIC ACID HCL 4-AMINO-TETRAHYDRO-PYRAN-4-CARBOXYLIC ACID 4-Aminotetrahydropyran-4-carbonitrile Hydrochloride (R)-3-Aminobutanenitrile Hydrochloride 3-((Dimethylamino)methyl)-5-methylhexan-2-one oxalate 5-Bromo-2-nitropyridine 4-(Dimethylamino)tetrahydro-2H-pyran-4-carbonitrile Diclofenac Potassium Ornidazole IP Diclofenac Sodium IP/BP/EP/USP Mefenamic Acid IP/BP/EP/USP Aceclofenac IP/BP/EP Nimesulide BP TIN AAS SOLUTION SILVER AAS SOLUTION ANDRADES REAGENT SOLUTION GRAMS SAFRANINE STAINING SOLUTION PLATINUM AAS SOLUTION TUNGSTEN AAS SOLUTION SODIUM METHYL PARABEN Methylcobalamin (vitamin B12) SODIUM VALPROATE Racecadotril XANTHAN GUMRelated products of tetrahydrofuran

![(E)-3-[[[3-[2-(7-CHLORO-2-QUINOLINYL)ETHENYL]PHENYL]-[[(3-DIMETHYLAMINO)-3-OXOPROPYL]THIO]METHYL]THIO]-PROPANOIC ACID, SODIUM SALT](https://img.chemicalbook.in/CAS/GIF/115104-28-4.gif)

You may like
-
 TOFA CAS 54857-86-2View Details
TOFA CAS 54857-86-2View Details
54857-86-2 -
 ethyl 2-(3-(tert-butyl)phenoxy)-2-methylpropanoate 98%View Details
ethyl 2-(3-(tert-butyl)phenoxy)-2-methylpropanoate 98%View Details -
 89796-99-6 Aceclofenac IP/BP/EP 98%View Details
89796-99-6 Aceclofenac IP/BP/EP 98%View Details
89796-99-6 -
 61-68-7 98%View Details
61-68-7 98%View Details
61-68-7 -
 Diclofenac Sodium IP/BP/EP/USP 98%View Details
Diclofenac Sodium IP/BP/EP/USP 98%View Details
15307-79-6 -
 Ornidazole IP 16773-42-5 98%View Details
Ornidazole IP 16773-42-5 98%View Details
16773-42-5 -
 51803-78-2 Nimesulide BP 98%View Details
51803-78-2 Nimesulide BP 98%View Details
51803-78-2 -
 15307-81-0 98%View Details
15307-81-0 98%View Details
15307-81-0