5-METHYL-3-HEPTANONE
Synonym(s):5-Methyl-3-heptanone;Ethyl amyl ketone, Ethyl isoamyl ketone, Isoamyl ethyl ketone;Ethyl isoamyl ketone
- CAS NO.:541-85-5
- Empirical Formula: C8H16O
- Molecular Weight: 128.21
- MDL number: MFCD00009340
- EINECS: 208-793-7
- SAFETY DATA SHEET (SDS)
- Update Date: 2024-12-18 14:15:30
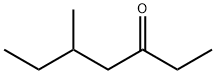
What is 5-METHYL-3-HEPTANONE?
Description
Ethyl amyl ketone is a colorless liquid with amild, fruity odor. Molecular weight = 128.24; Boilingpoint = 157.2℃; Freezing/Melting point = - 56.7℃; Vaporpressure = 2 mmHg at 20℃; Flash point = 57℃ (oc). HazardIdentification (based on NFPA-704 M Rating System): Health0, Flammability 2, Reactivity 0. Insoluble in water
Chemical properties
CLEAR LIGHT YELLOW LIQUID
Chemical properties
5-Methyl-3-heptanone is a colorless liquid of low volatility. It has an agreeable penetrating odor that resembles the essence of apricots and peaches. Threshold odor concentrations of 6 and ,5 ppm have been reported .
The Uses of 5-METHYL-3-HEPTANONE
- 5-Methyl-3-heptanone can be converted into the corresponding gem-dihydroperoxide using aqueous hydrogen peroxide and chlorosulfonic acid as a catalyst at room temperature.
- It can be reduced to its corresponding alcohol using sodium borohydride in urea/choline chloride eutectic salt.
- It can undergo condensation with tert-butyl-sulfinamide to form the corresponding chiral tert-butyl-sulfinyl-ketimine, which can further react with ylides derived from trimethylsulfonium iodide and S-allyl tetrahydrothiophenium bromide to form highly substituted chiral aziridines.
The Uses of 5-METHYL-3-HEPTANONE
Ethyl amyl ketone is used as a solvent forvinyl resins and nitrocellulose resins.
The Uses of 5-METHYL-3-HEPTANONE
Solvent for resins; organic intermediate
Production Methods
U.S. production and importation of 5-methyl-2-heptanone was estimated to be relatively low (<25,000 lb at a single site) in 2005 as data for 5-methyl-2-heptanone were not included in the 2006 U.S. EPA Inventory Update Reporting database.
Health Hazard
Exposure to ethyl amyl ketone can causeirritation of the eyes, nose, and skin. Athigh concentrations its exposure can leadto ataxia, prostration, respiratory pain, andnarcosis. A 5-minute exposure to 50 ppmmay produce a mild irritating effect on theeyes and nose in humans. Inhalation of3000 ppm of ethyl amyl ketone for 4 hourswas highly toxic to mice, and 6000 ppm for8 hours was lethal to rats. The odor thresholdis 6 ppm.
Fire Hazard
Combustible liquid; flash point (open cup) 59°C (138°F); vapor density 2 torr at 25°C (77°F); fire-extinguishing agent: “alcohol” foam, dry chemical, or CO2; a water spray may be effective to cool it below its flash point. It forms explosive mixtures with air at elevated temperatures; the LEL and UEL values are not reported.
Flammability and Explosibility
Flammable
Safety Profile
Moderately irritating to skin, eyes, and mucous membranes by inhalation and ingestion. Narcotic in high concentration. Flammable liquid when exposed to heat, sparks, or flame. When heated to decomposition it emits acrid smoke. To fight fire, use foam, CO2, dry chemical. See also KETONES.
Potential Exposure
PrimaryIrritant. Ethyl amyl ketone is used as a solvent for resins, inthe manufacture of perfume, and as an organicintermediate
First aid
If this chemical gets into the eyes, remove anycontact lenses at once and irrigate immediately for at least15 min, occasionally lifting upper and lower lids. Seek medical attention immediately. If this chemical contacts theskin, remove contaminated clothing and wash immediatelywith soap and water. Seek medical attention immediately. Ifthis chemical has been inhaled, remove from exposure,begin rescue breathing (using universal precautions, including resuscitation mask) if breathing has stopped and CPR ifheart action has stopped. Transfer promptly to a medicalfacility. When this chemical has been swallowed, get medical attention. Give large quantities of water and inducevomiting. Do not make an unconscious person vomit.
Environmental Fate
Chemical/Physical. 5-Methyl-3-heptanone will not hydrolyze because it does not contain a hydrolyzable functional group.
storage
Color Code—Red: Flammability Hazard: Store ina flammable liquid storage area or approved cabinet awayfrom ignition sources and corrosive and reactive materials.Prior to working with this chemical you should be trainedon its proper handling and storage. Ethyl amyl ketone mustbe stored to avoid contact with oxidizers (such as perchlorates, peroxides, chlorates, nitrates, and permanganates)since violent reactions occur. Store in tightly closed containers in a cool, well-ventilated area. Use only nonsparkingtools and equipment, especially when opening and closingcontainers of ethyl amyl ketone. Wherever ethyl amylketone is used, handled, manufactured, or stored, use explosion-proof electrical equipment and fittings
Shipping
This compound requires a shipping label of“FLAMMABLE LIQUID.” It falls in Hazard Class 3 andPacking Group II
Incompatibilities
Forms explosive mixture with air.Contact with oxidizers may cause fire and explosions.Incompatible with strong bases, reducing agents, aldehydes,nitric acid, aliphatic amines
Waste Disposal
Ethyl amyl ketone wastes can be destroyedby incineration or by treatment with moltenmetal salts.
Properties of 5-METHYL-3-HEPTANONE
| Melting point: | -56.65°C |
| Boiling point: | 157-162 °C (lit.) |
| Density | 0.823 g/mL at 25 °C (lit.) |
| vapor pressure | 2.7 hPa (25 °C) |
| refractive index | n |
| Flash point: | 111 °F |
| storage temp. | Store below +30°C. |
| solubility | 3g/l |
| form | Colorless liquid |
| color | Colorless liquid with a fruity odor |
| Odor | at 100.00 %. herbal sweet oily |
| explosive limit | 0.9-48%(V) |
| Water Solubility | 1.92g/L at 19.9℃ |
| Merck | 14,6084 |
| BRN | 506345 |
| Henry's Law Constant | 1.30 x 10-4 atm?m3/mol at 20 °C (approximate - calculated from water solubility and vapor pressure) |
| Exposure limits | TLV-TWA 130 mg/m3 (25 ppm) (ACGIH). |
| CAS DataBase Reference | 541-85-5(CAS DataBase Reference) |
| EPA Substance Registry System | 5-Methyl-3-heptanone (541-85-5) |
Safety information for 5-METHYL-3-HEPTANONE
| Signal word | Warning |
| Pictogram(s) |
 Flame Flammables GHS02  Exclamation Mark Irritant GHS07 |
| GHS Hazard Statements |
H226:Flammable liquids H319:Serious eye damage/eye irritation H335:Specific target organ toxicity, single exposure;Respiratory tract irritation |
| Precautionary Statement Codes |
P210:Keep away from heat/sparks/open flames/hot surfaces. — No smoking. P305+P351+P338:IF IN EYES: Rinse cautiously with water for several minutes. Remove contact lenses, if present and easy to do. Continuerinsing. |
Computed Descriptors for 5-METHYL-3-HEPTANONE
5-METHYL-3-HEPTANONE manufacturer
Herbochem Industries
New Products
(S)-3-Aminobutanenitrile hydrochloride 4-Methylphenylacetic acid N-Boc-D-alaninol N-BOC-D/L-ALANINOL Tert-butyl bis(2-chloroethyl)carbamate 3-Morpholino-1-(4-nitrophenyl)-5,6-dihydropyridin- 2(1H)-one Furan-2,5-Dicarboxylic Acid Tropic acid 1-Bromo-3,5-Di-Tert-Butylbenzene S-2-CHLORO PROPIONIC ACID ETHYL ISOCYANOACETATE 2-Bromo-1,3-Bis(Dimethylamino)Trimethinium Hexafluorophosphate 4-IODO BENZOIC ACID 3-NITRO-2-METHYL ANILINE 1-(2,4-DICHLOROPHENYL) ETHANAMINE (2-Hydroxyphenyl)acetonitrile 4-Bromopyrazole 2-(Cyanocyclohexyl)acetic acid 4-methoxy-3,5-dinitropyridine 1-(4-(aminomethyl)benzyl)urea hydrochloride 2-aminopropyl benzoate hydrochloride diethyl 2-(2-((tertbutoxycarbonyl)amino) ethyl)malonate tert-butyl 4- (ureidomethyl)benzylcarbamate Ethyl-2-chloro((4-methoxyphenyl)hydrazono)acetateRelated products of tetrahydrofuran
You may like
-
 541-85-5 / 57968-70-4 5-Methyl-3-heptanone 98%View Details
541-85-5 / 57968-70-4 5-Methyl-3-heptanone 98%View Details
541-85-5 / 57968-70-4 -
 5-Methyl-3-heptanone CAS 541-85-5View Details
5-Methyl-3-heptanone CAS 541-85-5View Details
541-85-5 -
 5-Methyl-3-heptanone CAS 541-85-5View Details
5-Methyl-3-heptanone CAS 541-85-5View Details
541-85-5 -
 5-Methyl-3-heptanone CAS 541-85-5View Details
5-Methyl-3-heptanone CAS 541-85-5View Details
541-85-5 -
 1975-50-4 98%View Details
1975-50-4 98%View Details
1975-50-4 -
 2-HYDROXY BENZYL ALCOHOL 98%View Details
2-HYDROXY BENZYL ALCOHOL 98%View Details
90-01-7 -
 14714-50-2 (2-Hydroxyphenyl)acetonitrile 98+View Details
14714-50-2 (2-Hydroxyphenyl)acetonitrile 98+View Details
14714-50-2 -
 118753-70-1 98+View Details
118753-70-1 98+View Details
118753-70-1