L(-)-Carvone
Synonym(s):Carvol;(?)-p-Mentha 6,8-diene 2-one;(?)-Carvone;(R)-(?)-Carvone;(R)-5-Isopropenyl-2-methyl-2-cyclohexenone
- CAS NO.:6485-40-1
- Empirical Formula: C10H14O
- Molecular Weight: 150.22
- MDL number: MFCD00001578
- EINECS: 229-352-5
- SAFETY DATA SHEET (SDS)
- Update Date: 2025-12-17 09:49:25
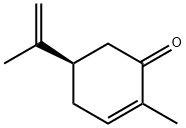
What is L(-)-Carvone?
Description
November is flavor and aroma month at MOTW!
Like limonene, the Molecule of the Week for November 1, 2021, carvone is a monoterpene that exists in nature as two enantiomers: (R)-carvone [aka (–)-carvone, L-carvone] and (S)-carvone [aka (+)-carvone, D-carvone]. The (R)-isomer is shown here.
Both enantiomers have well-recognized flavors and aromas. (R)-carvone has the flavor and aroma of mint; it is the dominant compound in spearmint oil. Like (R)-limonene, (S)-carvone1 is a key component of caraway and dill seed oils.
In 1841, Swiss chemist Eduard Schweizer isolated what eventually became known as (S)-carvone from oil extracted from caraway seeds (Carum carvi), from which carvone gets its name. Fifty years later, German chemist W. Kwaenick discovered its enantiomer in the oils of spearmint (Mentha spicata) and kuromoji (Lindera umbellata, a deciduous Asian shrub). In 1905, H. Waldbaum and O. Hüthig at what is now Technische Universit?t Dresden (Germany) reported that both isomers exist in gingergrass (Paspalum distichum) oil.
Both carvones are commercial products used in a variety of industries, primarily for their flavors and aromas. Natural (R)-carvone is used to flavor chewing gum and mint candies and to provide aromas in personal-care products, air fresheners, and aromatherapy oils. In 2009, the US Environmental Protection Acency registered it for use in insect repellents.
(S)-Carvone has fewer uses. Its presence in caraway oil adds to the flavor of kümmel, a liqueur popular in Europe. It is used in agriculture to prevent the premature sprouting of potatoes. Both isomers are used as starting materials for synthesizing complex terpenoids.
At 3800 t/year, the worldwide market for (R)-carvone is much larger than that of its (S)-isomer (10 t/year). Much (R)-carvone is extracted from natural mint, but about half of the commercial product is synthesized from (R)-limonene.
1. CAS Reg. No. 2244-16-8.
Chemical properties
clear colorless to pale yellow liquid
Chemical properties
Caravone occurs in different forms. l-Carvone exhibits odor of spearmint, while d-carvone exhibits odor reminiscent of caraway.
Occurrence
R-Carvone is the main substance in spearmint oil, also present in toothpastes; it is the cause of delayed-type allergy resulting in cheilitis, but Hansson et al. also described a case of angioedema of the lips appearing within minutes after contact with toothpaste, with an open test resulting in an immediate and strong reaction to carvone.
The Uses of L(-)-Carvone
(R)-(-)-Carvone is used in the flavor and food industry such as chewing gum additives. It is used in air freshening products such as in essential oils as well as in aromatherapy and alternative medicine. It is used to prepare carvomenthol, carvomenthone, dihydrocarvone, carveol and limonene. It reacts with lithium dimethylcuprate to place a methyl group trans to the isopropenyl group with good stereoselectivity. It is also used as a mosquito repellent and prevent premature sprouting of potatoes. Further, it is employed as an important starting material for the synthesis of enantiopure (R)-(+)-3-methyl-6-isopropenyl-cyclohept-3-enone-1 and (4S,6R,7R)-trihydroxy-1-octyne derivatives. It is utilized as a vital raw material for the asymmetric total synthesis of natural products. In addition to this, it is used as a chiral starting material.
Definition
ChEBI: A carvone having (R) configuration.
Aroma threshold values
Detection: d-Carvone: 6.7 to 820 ppb; l-carvone: 2.7 to 600 ppb
General Description
(-)-Carvone, a monoterpene ketone which is the main active component of mentha plant species like Mentha spicata. It has antinociceptive activity which is found to be associated with decreased peripheral nerve excitability.
Safety Profile
Poison by intravenous route. Moderately toxic by ingestion. When heated to decomposition it emits acrid smoke and irritating fumes.
Synthesis
Carvone occurs in the dextro, levo and racemic form; l-carvone can be isolated from the essential oil of spearmint or is commercially synthesized from d-limonene; d-carvone is usually prepared by fractional distillation of oil of caraway, also from dillseed and dillweed oils, but this type differs in odor and flavors.
Properties of L(-)-Carvone
| Melting point: | 25°C |
| Boiling point: | 227-230 °C (lit.) |
| alpha | -57 º (neat) |
| Density | 0.959 g/mL at 25 °C (lit.) |
| vapor density | 5.2 (vs air) |
| vapor pressure | 0.4 mm Hg ( 20 °C) |
| refractive index | n |
| FEMA | 2249 | CARVONE |
| Flash point: | 192 °F |
| storage temp. | Keep in dark place,Inert atmosphere,2-8°C |
| solubility | Chloroform, Methanol |
| appearance | colorless to pale yellow liquid |
| form | Liquid |
| color | Clear colorless to pale yellow |
| Odor | at 100.00 %. sweet spearmint herbal minty |
| optical activity | [α]20/D 61°, neat |
| Water Solubility | PRACTICALLY INSOLUBLE |
| JECFA Number | 380 |
| Merck | 14,1874 |
| BRN | 2206714 |
| Dielectric constant | 11.0(22℃) |
| CAS DataBase Reference | 6485-40-1(CAS DataBase Reference) |
| NIST Chemistry Reference | Carvone(6485-40-1) |
| EPA Substance Registry System | 2-Cyclohexen-1-one, 2-methyl-5-(1-methylethenyl)-, (5R)- (6485-40-1) |
Safety information for L(-)-Carvone
| Signal word | Warning |
| Pictogram(s) |
 Exclamation Mark Irritant GHS07 |
| GHS Hazard Statements |
H302:Acute toxicity,oral H317:Sensitisation, Skin |
| Precautionary Statement Codes |
P261:Avoid breathing dust/fume/gas/mist/vapours/spray. P264:Wash hands thoroughly after handling. P264:Wash skin thouroughly after handling. P270:Do not eat, drink or smoke when using this product. P280:Wear protective gloves/protective clothing/eye protection/face protection. P301+P312:IF SWALLOWED: call a POISON CENTER or doctor/physician IF you feel unwell. P302+P352:IF ON SKIN: wash with plenty of soap and water. |
Computed Descriptors for L(-)-Carvone
| InChIKey | ULDHMXUKGWMISQ-SECBINFHSA-N |
L(-)-Carvone manufacturer
New Products
4,4-Difluoropiperidine hydrochloride tert-butyl 9-methoxy-3-azaspiro[5.5]undecane-3-carboxylate Indole Methyl Resin N-Isopropylurea N,N-Dicyclohexylcarbodiimide(DCC) MELDRUMS ACID 5-METHYLISOXAZOLE-4-CARBOXYLIC ACID Magnessium Bis glycinate Zinc ascorbate 1-bromo-2-butyne 2-acetamidophenol 9(10H)-anthracenone Erythrosin B, 4-Piperidinopiperidine 2-((4-morpholinophenylamino) (methylthio) methylene) malononitrile 2,4-dihydroxybenzaldehyde 3-(4-morpholinophenylamino)-5-amino-1H-pyrazole-4-carbonitrile Methyl 2-methylquinoline-6-carboxylate 2,6-dichloro-4-nitropyridine 4-Bromo-2-chlorobenzonitrile 2-(benzylamino)acetic acid hydrochloride 4-(tert-Butoxycarbonylamino)but- 2-ynoic acid 3,4-dihydro-2H-benzo[b][1,4]dioxepine 1-Phenyl-1-cycloprppanecarboxylicacidRelated products of tetrahydrofuran

You may like
-
 L-Carvone 98%View Details
L-Carvone 98%View Details
6485-40-1 -
 6485-40-1 99%View Details
6485-40-1 99%View Details
6485-40-1 -
 L-Carvone 6485-40-1 98%View Details
L-Carvone 6485-40-1 98%View Details
6485-40-1 -
 (R)-(-)-Carvone CAS 6485-40-1View Details
(R)-(-)-Carvone CAS 6485-40-1View Details
6485-40-1 -
 L(-)-Carvone 98% (HPLC) CAS 6485-40-1View Details
L(-)-Carvone 98% (HPLC) CAS 6485-40-1View Details
6485-40-1 -
 (R)-(−)-Carvone CAS 6485-40-1View Details
(R)-(−)-Carvone CAS 6485-40-1View Details
6485-40-1 -
 L Carvone (Aroma Chemicals)View Details
L Carvone (Aroma Chemicals)View Details
6485-40-1 -
 L(-)-Carvone 98+View Details
L(-)-Carvone 98+View Details
6485-40-1
